Introduction
Mexico is one of the top leaders in mango production. Around 193.343 thousand hectares for cultivation have been established. In 2018, Mexico reported total production of 2 million tons of mango with an income of 3,992 million pesos. Exports represent an annual income of 155 million dollars (SAGARPA, 2018). Moreover, mango contains phenolic compounds and carotenoids that have been linked to beneficial effects against noncommunicable diseases due to its antioxidant activity (AOA) against free radicals such as hydroxyl, superoxide and singlet oxygen (Mesa-Vanegas et al., 2010). However, mango fruits are only available for a short period because it is seasonal and perishable, which is why large post-harvest and economic losses are generated. The current market demands processed foods with nutritional and sensorial properties similar to fresh foods. The use of thermal technologies causes a significant loss of nutrients and flavors, affecting the organoleptic quality of the final product (Zhao et al., 2013). Therefore, the food industry is looking for new preservation technologies to guarantee food safety and avoid negative changes in sensory, nutritional, physicochemical and antioxidant characteristics. Currently, non-thermal technologies are being developed for food processing, such as high hydrostatic pressure (HHP). This technology has great acceptance because food quality is minimally modified (Oey et al., 2008). It is based on physical methods on the principles of Le Chatelier and isostatic, without requiring high temperatures. HHP consists of applying high pressure to solids or liquid foods, where the fluid transmitter is usually water. Originally, HHP was used to inactivate microorganisms, since it has an effect on cell morphology, modifies enzymatic reactions and can affect the genetic mechanisms of microbial cells without altering the sensory quality or nutrients of food (Cheftel,1995). Other factors such as the composition of the food matrix, water activity, and pH affect or enhance the effect of HHP. The microorganisms are more susceptible in a non-nutritive medium than in an enriched one, which exerts a protective effect due to the presence of proteins and vitamins as nutrients, as well as a higher viscosity (Reyes et al., 2011). Citrus juices were the first products subjected by HHP. The acid pH facilitates the destruction of microorganisms and avoids spore germination after treatment. The process with HHP, unlike thermal processes, allows preserving the taste of fresh juice and its nutritional content (Ferrari et al., 2010). The use of HHP up to 400 MPa is increasing in the food industry since it can be used as an alternative to traditional methods such as sterilization or pasteurization (Raventós-Santamaría, 2005). The potential use of HHP in biotechnological processes has been reported (Rivalain et al., 2010), specifically to decrease and/or eliminate enzymatic activity (Huang et al., 2013), microbial (Klotz et al., 2010) as well as to stabilize food (Jacobo-Velázquez et al., 2012). The use of HHP has been reported in fruit juices such as passion fruit (Laboissière et al., 2007), cucumber (Zhao et al., 2013), apple (Moody et al., 2014) and in mango juices for the destruction of Escherichia coli O157: H7 and some enzymes (Hiremath & Ramaswamy, 2012; Bermúdez-Aguirre et al., 2011). The quality of food products is affected by the inactivation or activation of some enzymes by using HPP. For example, E. coli dehydrogenases are completely inactivated when 100 MPa for 15 min at 27 °C is applied, while the activity of the aspartase of this bacterium increases at 680 MPa (Téllez-Luis et al., 2011). Hendrickx et al. (1998) grouped in two the HHP effects on the enzymes: relatively low pressures, 100 MPa that activate some enzymes, fundamentally those of monomeric type, and high pressures >600 MPa, which provoke enzymatic inactivation.
On the other hand, fresh unpasteurized fruit and vegetable juices are considered as a rising vehicle of foodborne diseases (Parish, 1997). In the absence of a specific pathogen-product association, the National Advisory Committee on Microbiological Criteria in Foods (NACMCF) (Buchanan, 1997) recommends the use of Escherichia coli O157:H7 or Listeria monocytogenes as reference organisms in the processing of juices, because they are the most difficult organisms to control due to their resistance to the acidity of the juice and limited heat lethality. Therefore, the objective of the present study was to evaluate the inactivation of E. coli in mango nectar applying HHP as well as to evaluate the effect of this unit operation on the main aromatic profile compounds and some physicochemical parameters with the purpose of optimizing the process for obtaining mango nectar.
Material and Methods
Nectar obtention
In order to elaborate the nectar, mango (Mangifera indica L.) ‘Ataulfo’ variety in consumption maturity was used. Fruits were disinfected and blanched to inactivate enzymes such as polyphenol oxidase, peroxidase, polygalacturonase, and catalase. The pulp was standardized with water in the proportion established by the Codex Alimentarius (25 % v/v) and carrying out a Regularization of Sugar (RA) at 13.5° Brix (Eq. 1).
Nectar was stored at -20 °C in order to ensure the conservation of the product.
Inoculum Preparation
Escherichia coli 0157: H7 ATCC 25922 resistant to rifampicin, isolated and previously studied by Castro-Rosas et al. (2012) was donated to Instituto Tecnológico de Tepic. The strain was activated on tripcasein soy agar added with rifampicin at 100 mg/L and incubated for 48 h at 35 °C. Dilutions were performed to obtain 1x106 CFU/ mL using the Neubauer chamber.
HHP treatment
100 mL of the inoculated nectar was packed (FoodSaver® ROL28 high vacuum bags), sealed to high vacuum in a V3835 sealing machine (FoodSaver®, USA) and processed in the CIP 42260 isostatic press (Quintus, Technologies, Ohio, USA) according with the protocol reported by Ruiz-Montañez et al. (2014). We used a 33 factorial design. Pressure (150, 200 and 250 MPa), temperature (25, 35 and 45 °C) and residence time (0, 10 and 20 min). The time zero min was considered as the time necessary for the samples to reach the indicated pressure in the HHP equipment (come up time, CUT=2.65 min). After the treatments, samples were stored at 4 °C for 6 days.
Inactivation of Escherichia coli 0157: H7
With the aim to determine the inactivation of E. coli, the surviving cells were counted after applying HHP. Decimal dilutions were carried out in isotonic solution at 0.85 % NaCl and then plated on ATBS agar added with 100 mg/L of rifampicin. The plates were incubated in triplicate at 35 °C from 24 to 48 h. The inactivation was calculated as the difference between the logarithms of the colony counts of the inoculated samples and the inoculated samples treated (log N0 - log N).
Identification and quantification of volatile compounds by SPME-CG-MS
The sample was prepared by placing 10 mL of mango nectar in a 20 mL vial, sealed with a septum and then homogenized with 1 g of NaCl (1:10) on a magnetic stirrer model C-MAG H57. Next, the sample was incubated at 40 °C for 30 min, achieving the first thermodynamic equilibrium between the headspace and the sample. After this time, the solidphase microextraction fiber was inserted (SPME Fiber Assembly 65 m PDMS/DVB, Fused Silica 24Ga, Manual Holder, 3pk, SUPELCO), exposing it in the headspace inside the vial for 20 min. In this time, a second thermodynamic equilibrium is reached, in which the aromatic compounds adsorbed on the fiber are a representative sample of the contents in the headspace. Finally, the SPME fiber was extracted and immediately inserted into the injection port, exposing the fiber for 4 min for the desorption of the aromas (Solís-Solís et al., 2007). The quantification of mango nectar aromatic compounds was carried out in a gas chromatograph CP3800 (VARIAN, USA), with manual injection, equipped with a flame ionization detector (FID), fitted with a capillary column WCOT fused silica, 3000 x 0.25 mm, covered with CP-SIL 5CB (thickness 25 μm). Nitrogen was used as a carrier gas with a flow rate of 1 mL/min. The operating conditions of the gas chromatograph were as follows: 250 °C injector temperature, initial oven temperature 40 °C increasing to 200 °C at a heating rate of 3 °C/min. Subsequently, the temperature was increased again up to 230 °C, with a heating rate of 5 °C/ min as previously reported by Solís-Solís et al. (2007).
The identification of the aromatic compounds was performed in a gas chromatograph 7890A (Agilent Technologies, California, USA); coupled to a 240 Ion Trap GC/MS mass spectrum (Agilent Technologies, California, USA), using helium as a carrier gas at a flow rate of 1 mL/min and an RFX-SSil MS column (3000 x 0.25mm DIx0.25μm Film). The transfer line was preserved at 250 °C; the mass spectra were scanned at 70 eV in the m/z range of 60 - 600 mass units. The injection was done manually using the SPME fiber. Volatile compounds were identified by comparing the linear retention rates and the mass spectrum data with data from the literature and with the data bank (NIST/49 K Mass Spectral Database, Hewlett-Packard Co., Palo Alto, CA USA and Mass Spectral Data Record with Structures, Wiley 6.1, NY, USA).
Physicochemical characterization
The total soluble solids (SST) were measured with a digital refractometer NAR-T1 (ATAGO, USA). Hydrogen Potential (pH) was measured using a pH meter HI18653 (Hanna, Italy). All measurements were made in triplicate.
Sensory analysis
Sensory analysis was performed with 26 untrained judges using a triangular preference test (Pedrero & Pang, 1997). The data were analyzed using an ANOVA and SAS system software version 10.0 (p>0.05).
Results
Inactivation of Escherichia coli O157: H7 by HHP
The logarithmic reduction values of E. coli in pressurized mango nectar stored at 4 °C for 6 days showed a directly proportional relationship between the pressure and the lethality of the bacteria (Table 1). At 250 MPa, a greater logarithmic reduction is obtained in comparison with the treatments at 200 and 150 MPa (p<0.05). HHP induces changes in the cell, the main damage occurs on the cell membrane, structure responsible for regulating the entry and exit of nutrients, and constituted by lipids that are particularly sensitive to the effects of pressure, but more resistant than proteins (Vázquez-Gutiérrez et al., 2011). The increase in pressure augments the order of the hydrocarbon chains, as well as the phase transition temperature of the membrane in the gel state to the liquid crystalline state (Bartlett, 2002).
Table 1 Inactivation of Escherichia coli O157: H7 (Log CFU/mL) in mango nectar treated by high hydrostatic pressures and stored at 4 °C.
| Pressure | Temperature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | ||||||||
| 0 min | 10 min | 20 min | 0 min | 10 min | 20 min | 0 min | 10 min | 20 min | ||
| 150MPa | Day 0 | 2.8 | 3.96 | 4.1 | 6 | 6 | 6 | 6 | 6 | 6 |
| Day 2 | 3.5 | 3.01 | 3.5 | 5.5 | 5 | 5.5 | 6 | 6 | 6 | |
| Day 4 | 3.5 | 2.57 | 3.6 | 5.2 | 5 | 5.2 | 6 | 6 | 6 | |
| Day 6 | 3.2 | 2.44 | 3.3 | 5.2 | 5 | 5.2 | 6 | 6 | 6 | |
| 200MPa | Day 0 | 3.9 | 4.46 | 4.3 | 6 | 6 | 6 | 6 | 6 | 6 |
| Day 2 | 3.8 | 3.95 | 4.3 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Day 4 | 3.8 | 4.23 | 4.6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Day 6 | 3.7 | 3.93 | 4.4 | 6 | 6 | 6 | 6 | 6 | 6 | |
| 250MPa | Day 0 | 4.7 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Day 2 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Day 4 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Day 6 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
The Food and Drug Administration (FDA) has proposed a reduction of 5-log in pathogenic microorganisms (move to discussion). In this study, using 150 MPa at 45 °C E.coli (6 Log) was completely inactivated. The same inhibition was accomplished using 200 MPa at 35 °C. 250 MPa at 25 °C for 10 min completely inactivate E. coli. These results satisfy the parameters established by the FDA. The statistical analysis for logarithmic reduction indicated that time, temperature and pressure significantly affect the inhibition process (p<0.05).
On the day of processing (storage time 0), the effect of 150, 200 and 250 MPa pressures at room temperature showed different levels of inactivation. In the range from 20 to 180 MPa, microbial growth is delayed, and protein synthesis is also inhibited. The loss viability of the cells starts from pressures of approximately 180 MPa, once this pressure increases, the rate of inactivation increases exponentially. Damage to the integrity of the membrane and the denaturation of a protein is observed when higher pressures are applied (Vázquez-Gutiérrez et al., 2011). It should be noted that the different effects exerted by HHP on microorganisms directly depend on the stage of development in which they are since cells in logarithmic phase have a greater sensitivity to treatment by HHP than those in stationary phase. Mañas & Mackay (2004) observed this behavior using Escherichia coli J1 strain in an exponential and stationary phase. Several cellular modifications were also observed such as aggregation of cytoplasmic proteins and condensation of the nucleotides when the pressures were equal or greater than 200 MPa. This may be due to the microorganisms being in cell division during the logarithmic phase and therefore, the membrane is more sensitive to external factors (Ayvaza et al., 2012).
The irreversible protein denaturation under pressure greater than 300 MPa corresponds to the range of pressure necessary to inactivate vegetative cells (Tabilo-Munizaga et al., 2014). In this study, temperatures of 35 and 45 ºC were applied (moderate temperatures) to diminish the possibilities of survival of the microorganism. In addition, it has been reported that the response to pressure stress at 53 MPa in the genus Escherichia coli induces the synthesis of 55 proteins, including 11 thermal shock proteins and 4 cold shock proteins as a defense mechanism of the cell (Buzrul et al., 2008). The pressure is the only stress factor that can simultaneously induce the synthesis of heat shock and cold shock proteins (Ambrosi et al., 2016). One of the effects of the HHP treatments is the breakdown of non-covalent bonds (ionic, hydrophobic, hydrogen bonds) of the proteins and therefore, the secondary, tertiary and quaternary structure can unfold and dissociate, while the primary structure remains unchanged (Dzwolak et al., 2002). According to Messens et al. (1997) applying a pressure lower than 150 MPa changes in the quaternary structure are observed. Nevertheless, it is necessary to apply more than 200 MPa to significantly modify the secondary and tertiary structures. The induction of these proteins represents an attempt by E. coli to diminish the damaging effects of increased pressure on membrane integrity, translation processes, as well as the stability of macromolecules (Bartlett, 2002). For this reason, pressures of less than 300 MPa, temperature or prolonged times should be applied. The combined treatment avoids the possibility of having stressed cells that synthesize defense proteins and therefore the microorganism can survive and recover from sublethal damage during storage.
Regarding the effect of the residence time in the inactivation level, we observed that the inhibition increases from 2.8 to 4.1 log CFU/mL when the come up time is augmented, CUT=2.65 min at 20 min the treatment of 150 MPa at 25 °C. Some authors have suggested that increasing the treatment time for some cases, does not generate a better response in the case of the HHP system (Raventós-Santamaría, 2005). However, a high concentration of solutes above 30 % can induce a greater resistance of the microorganism to the pressure due to the physiological conditions of the organism when the water activity is reduced. This resistance may decrease with increasing temperature and time of treatment (Telléz-Luis et al., 2001).
The pressure does not generate significant effects until combined treatments with temperature are applied and in the case of 150 MPa with greater residence time (Table 1). Rivalain et al. (2010) report that the application of temperature (between 45 and 70 °C) synergistically improves the effectiveness of the pressure. Synergy is also generated by combining low pH with HHP. When the microorganism is present in an acid medium, as the Gramnegative bacteria, HHP produces an “electroporation” effect due to the stacking of the lipid compounds of the cell membrane, which means an empty space on the cell membrane. Taking this into account, it is not possible to regulate the entry of substances as well as hydrogen ions present in the juice, promoting cytoplasmic acidification and inducing subsequent cell death (Alpas et al., 2008; Vázquez-Gutiérrez et al., 2011).
Nectar used in this study showed an acid pH of 3.3 ± 0.1. The influence of the food components on the effect of pressure on microorganisms has been previously reported (Patterson, 2005). Seiji et al. (2004) obtained reductions of 2.5 log of Escherichia coli O157: H7 when applying 200 MPa for 20 min to the apple juice (pH 3.69). In this study, at those conditions of pressure and time, we accomplished a higher reduction (4.3 log CFU/mL) of E. coli at 25 ºC (Table 1). By evaluating the inactivation at different retention times it is possible to consider that the pH of the mango nectar, the temperature and the pressure of the treatment result in a synergistic effect causing a greater inactivation, which indicates that possibly a low pH increases the inactivation of the vegetative bacteria by high pressure (Van Opstal et al., 2005). It has been reported that this microorganism can survive in acidic foods up to a pH of 3.7 (Garlant-Miller & Kaspar, 1994). This value is higher than the mango nectar. When the pressure, temperature and acidic pH are combined, greater inhibition of bacteria is observed. By exposing the microorganism to a stress condition such as pressure, the microorganisms less baroresistant are inactivated. It is important to mention that baroduric microorganisms can survive to pressures of 200 MPa, but can not grow. Nevertheless, when applying a combined treatment with temperature a greater logarithmic reduction is observed, since synthesize proteins as defense response as responsible for the three stress conditions, seems to be complicated for the cell because an energy demand in the form of ATP would be necessary, which would be synthesized in a very limited way due to the denaturation by the pressure of the enzyme ATPase located in the cell membrane. Moreover, it is necessary to consider that during the pressurization process adiabatic heating is generated in the processed product, increasing the temperature from 2 to 3 °C per 100 MPa (Hernández-García, 2007), or from 3 to 9 °C per each 100 MPa (Patterson, 2005), depending on the initial temperature. Several enzyme systems of microorganisms are inhibited or inactivated by pressure. This is the case of several dehydrogenases in Escherichia coli (100 MPa), carboxypeptidases of yeasts (400 MPa) and ATPase located in the phospholipid layer involved in the phenomenon of active transport through the membrane. The Na+/ K+-ATPase activity of the cell membrane is reduced by high pressure. This effect is probably due to the associated bilayer (Chong et al., 1985). When the pressure is applied, several sites inside the bacterial cell can be damaged and therefore, no function of the ATPase can be performed due to direct denaturation or dislocation of the membrane. ATP is no longer hydrolyzed, and therefore, it is no longer available to carry out the active transport of protons, the cellular pH is acidified and the cell eventually dies.
Aromatic profile of mango nectar treated by HHP
In the 27 treatments of this study, ethanol, α-pimeno, myrcene, 3-carene were detected in mango nectar (with 98 % reliability), which has been reported as components of the aroma (Beaulieu & Lea, 2003; Pandit et al., 2009), as well as nonanoic acid. In the aromatic profile analysis (Table 2), we found that only the increase in temperature has an effect decreasing the concentration of some volatile compounds in the medium, such as α-pimeno and ethanol.
Table 2 Optimum conditions of the process for the conservation of mango nectar treated by high hydrostatic pressures.
| Compound | Factor | ||
|---|---|---|---|
| Temperature (ºC) | Pressure (MPA) | Time (min) | |
| Escherichia coli O157:H7 | 36.15 | 250.0 | 14.08 |
| α-Pimen | 25.0 | 225.3 | 20.0 |
| β-Myrceno | 45.0 | 250.0 | 8.0 |
| 3-Carene | 25.0 | 150.0 | 20.0 |
| Nonanoico acid | 31.0 | 150.2 | 20.0 |
| Global | 25.0 | 250.0 | 20.0 |
It is possible to affirm the non-alteration of the aroma in this food at different pressure levels because the high pressures do not affect the covalent bonds that are typical of the aroma, but the non-covalent ones as the hydrogen bonds typical of the tertiary structure of the proteins undergo important modifications. This is possible to explain since the distance of a covalent bond is very small 10 A°, the chemical nature of the link indicates that a hybridization of the sp3 orbits exits, which leads to a smaller distance between C-C, C-H, C-O, S-S, among others and when applying pressure, the distance between the elements is smaller. However, due to the sharing of electrons they are not affected by this reduction of space, so aromatic compounds are not affected at pressures less than 1000 MPa (Sangronis et al., 1997). Ethanol was found at an average concentration of 30.92 ± 18.57 μg/L and is only affected by the treatment temperature, being a lower concentration when applying treatments at 35 and 45 °C. This compound has been reported by Lebrun et al., 2008, in Keitt and Kent varieties, which is released during the ripening process in fruits. Also, α-pimeno is affected by temperatures (35 and 45 °C). This is a monoterpene compound product of the secondary metabolism of some fruits such as mango due to the hydrolysis of sugars. This has been reported by Salazar et al. (2007) in the mango variety Ataulfo, Kent and Keitt (Lebrun et al., 2008) and by Pandit et al. (2009) in 27 mango varieties and in Tommy Atkins by Moreno et al. (2010). Nonanoic acid has been identified in this study, this compound is not altered by treatment conditions. β-myrcene and 3-carene are monoterpenes on which the pressure, time or temperature showed no effect. β-myrcene is present in mango fruit at physiological maturity, it has been reported in different varieties Ataulfo (Salazar et al., 2007), Kent and Keitt in the states of physiological and consumer maturity (Lebrun et al., 2008) and in 27 varieties of mango (Pandit et al., 2009) and the 3- carene is a product of secondary metabolism produced by the hydrolysis of sugars.
Effect of HHP on the total soluble solids and on the hydrogen potential
The value of the total soluble solids (SST, °Brix) was 13.5 ± 0.22 (average), statistically equal value to the obtained in SSR for mango nectar without treatment, which indicates that the pressure, time and temperature do not affect the concentration of SST (Figure 1). The main sugars in the mango are mostly fructose, sucrose, glucose as well as maltose and xylose (Tharanathan et al., 2006). In these sugars, covalent bonds predominate, considered stable bonds under pressure conditions less than 1000 MPa. These types of links share one or more pairs of electrons in an orbit and are high energy (95 Kcal/mol), thus the application of pressure does not release enough energy to break them. It has been reported that grape juice subjected to HHP (500 MPa) showed no change in the content of total soluble solids (Zhao et al., 2013). In melon, at 800 MPa, a slight decrease in SST was observed (Wolbang et al, 2008). The effect on the total soluble solids is not the same when treating a juice or a fruit since the juices are foods where the chemical compounds and nutrients individually receive and transmit the pressure proportionally. Butz et al., (2002) reported when pressurizing sugars such as fructose, sucrose, glucose, they remain without noticeable changes until 21 days after treatment. The low pH in the juices contributes to having a longer storage period. The average pH of the samples treated by HHP was 3.2 ± 0.012, a statistically equal value to the pH of the mango nectar without treatment (Figure 2). The statistical analysis (R=0.902) showed no effect of pressure, time and temperature (p<0.05) hence the hydrogen potential value is not altered. Heremans (1995) mentioned a decrease in pH of 0.3 units per 100 MPa, but during the depressurization process, the pH returns to its initial level.
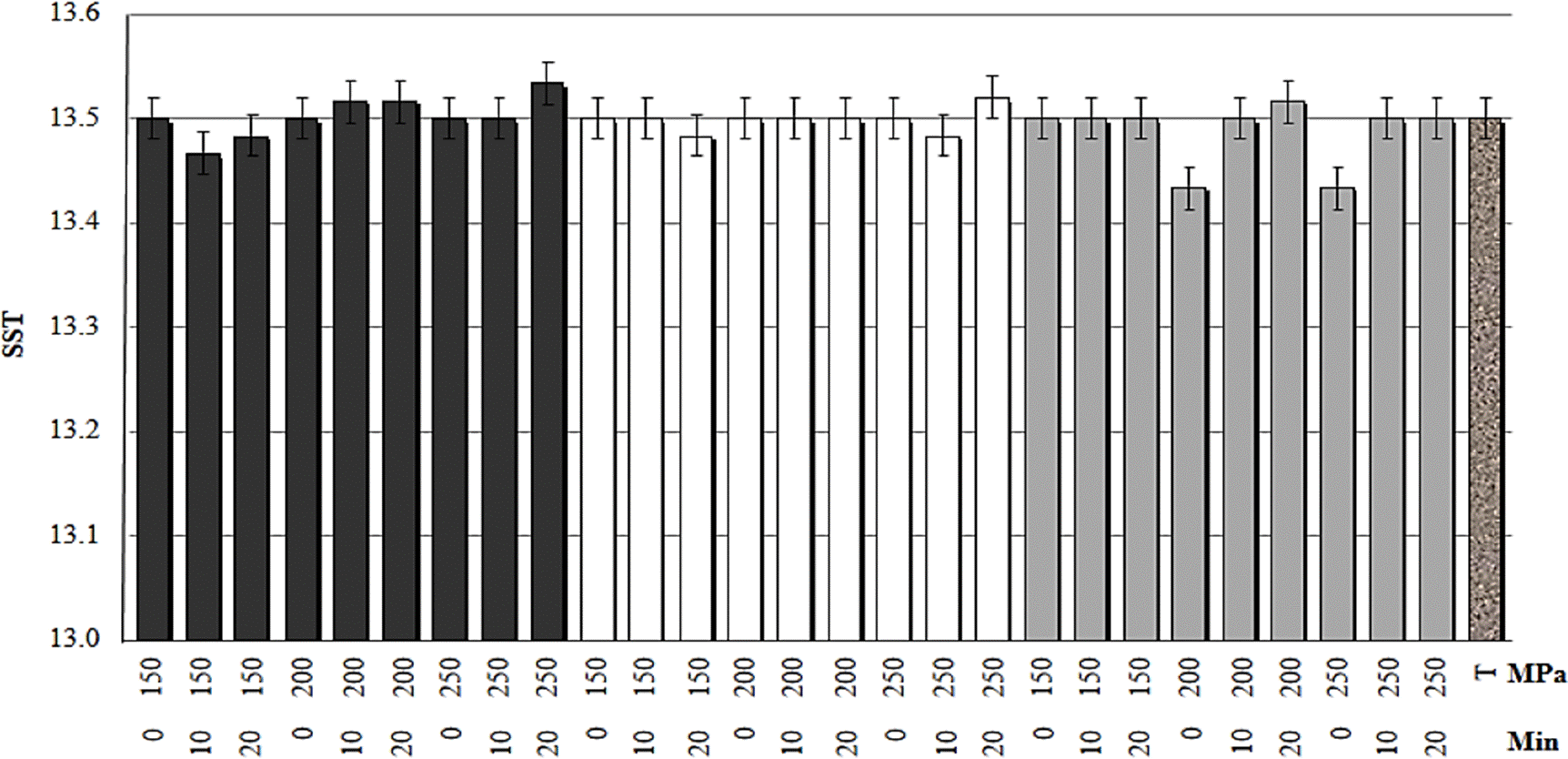
Figure 1 Effect of high hydrostatic pressure on the content of total soluble solids in mango nectar at different temperatures (black 25 °C, white 35 °C, gray 45 °C and T: control).
Process optimization
The optimal conditions for the inactivation of Escherichia coli O157:H7 to maximize the conservation of each of the aromatic compounds, independently, as well as the conditions for the integral conservation of mango nectar, evaluated in a global manner, are presented in Table 2. The total inhibition of Escherichia coli O157: H7 is possible from 250 MPa at 25 ºC for 10 min. However, the analysis of response surface, based on its calculation principles, determined as optimal conditions 250 MPa, 36.15 ºC, and 14.08 min. It is possible to visualize these conditions in the response surface (Figure 3), which shows in dark gray, the optimal zone for the inhibition of Escherichia coli O157:H7 under the conditions used in the different treatments.
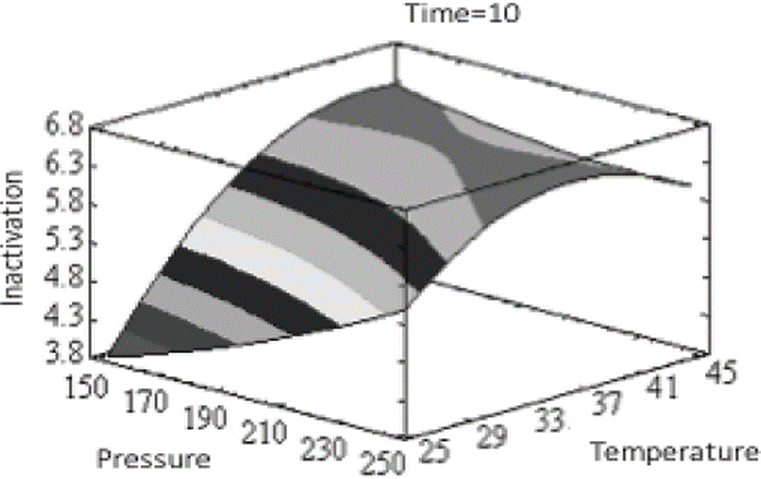
Figure 3 Response surface for obtaining the optimal conditions of the treatment of high hydrostatic pressures on the inhibition of Escherichia coli O157: H7.
Regarding the aromatic compounds evaluated in this study, we noticed by response surface analysis the effect of temperature above 35 ºC on the α-pimeno compound and ethanol, reporting as 25 ºC as the optimal temperature for this compound. This indicates that at low-temperature levels, the preservation of these compounds is guaranteed. We determined the optimal conditions of the process by applying the methodology of multiple responses in the response surface analysis considering the concentration of 3-carene, ethanol, β-myrcene, nonanoic acid, α-pimeno and logarithmic reduction of Escherichia coli O157:H7 (Table 2). It is important to mention that these conditions coincided with the optimal conditions for the inhibition of Escherichia coli O157:H7, which guarantees the preservation of the aromatic compounds of mango, as well as the nectar food safety.
Optimal conditions were applied to samples of mango nectar without inoculum of Escherichia coli O157:H7 in order to sensorially evaluate the differences between nectar treated with high pressures and nectar without treatment. The analysis of variance (p>0.05) allows us to state that the judges did not detect significant changes in the taste and smell of mango nectar treated with high hydrostatic pressures compared to the control without treatment.
Conclusions
The application of pressure, temperature and residence time was favorable in the inactivation of Escherichia coli O157: H7 in mango nectar. Complete inactivation was achieved with the treatment of 250 MPa, 25 °C and 10 min; the same results were obtained by applying 200 MPa, 35 °C and 150 MPa, 45 °C and 0 min. Soluble solids content and pH of mango nectar was no affected by the treatments. The increase of temperature in the treatments decreased the content of some volatile compounds such as α-pimeno and ethanol. The response surface analysis proposes as optimal process conditions for the conservation of the aromatic quality and the safety of the product 250 MPa, 25 ºC, and 20 min. HHP was effective on the inhibition of Escherichia coli O157:H7, without negatively affecting the properties and quality of mango nectar.











 texto en
texto en 



