Introduction
Currently, the study of dry and semiarid regions is of great importance, since adverse environmental conditions exist in these regions, such as drought, that limits the growth, production and survival of native trees and shrubs. A constant change of climate throughout years in these areas has fostered the incidence of extreme drought, causing a water deficit in the soil, and, in turn, a water stress in plants (Fleta-Soriano & Munné-Bosch, 2016). Under these conditions, plants experience a decrease in their water potentials in response to the lower water availability in the soil, negatively affecting its physiological and photosynthetic activity (Peña-Rojas et al., 2018) and reflecting a production reduction in biomass (Attia et al., 2015).
In the semiarid region of flatlands in northeastern Mexico, a marked climatic heterogeneity has favored the existence of a huge diversity of plant species (trees and shrubs), deciduous and evergreen, which present diverse growth patterns, as well as distinct foliar longevity and very varied phenology (González-Rodríguez et al., 2016). In this sense, trees and shrubs species coexisting in Tamaulipan thornscrub (TTS) have developed adaptation mechanisms, such as: foliar dimension and longevity; morphology; stomata dimension and density; leaflets abscission; foliar pubescence; thick and waxy cuticle, reduction in osmotic potential and resistance to water flow (González-Rodríguez et al., 2016). These characteristics have allowed plants to be able to minimize water loss by evapotranspiration (Striker, 2012), allowing them to maintain tissue water status (Donoso et al., 2015). The above mentioned is essential for native trees and shrubs species in northeastern Mexico to present adaptation physiological mechanisms (González-Rodríguez et al., 2016), because of water deficit and high temperatures (Warren et al., 2011), such as the reduction of foliar water potential as water availability decreases in soil until it is maintained over a threshold (Sánchez-Salguero et al., 2015). Therefore, the regulation of foliar water potential is one of the mechanisms that determines plants’ ability to survive in this type of regions (Fleta-Soriano & Munné-Bosch, 2016).
Studies performed by González-Rodríguez et al. (2011a, 2011b, 2016 and 2018), have attempted to directly link water potential (water status) presented by native species in response to environmental variables during drought periods in northeastern Mexico, in order to understand their ecophysiological response in presence of water deficit in the soil. In these studies, Celtis pallida, Forestiera angustifolia and Parkinsonia texana have been determined as the most tolerant species to water deficit. Thus, the objective of the present study was to determine the water status of four native plant species (Cordia boissieri, Prosopis laevigata, Helietta parvifolia and Karwinskia humboldtiana) and its relation with temperature, relative humidity, vapor pressure deficit, soil water content and precipitation. These species are of forage, forestry, medical and mainly ecological importance in TTS. Obtained results will be essential to determine which native species are more tolerant to drought and to be able to recommend their establishment in forestry plantations, reforestations or conservation works.
Material and Methods
The present research was performed in the experimental area of the Faculty of Forestry Sciences of the Autonomous University of Nuevo León, located at the coordinates 24°46’43’’ NL and 99°31’39’’ WL, which present an elevation of 370 masl. The climate is subtropical (Cfa) and semiarid (BSh), with presence of rains between the months from April to November and a drought period (heat wave) where temperatures can reach 45 °C; generally, average monthly temperature vary from 12.5 °C to 26.0 °C. Accumulated annual precipitation is of 800 mm. The type of soil for 0-40 cm depth is clayey silty, with an apparent density of 0.9 g·cm-3, pH of 7.60, an electrical conductivity of 0.14 dS· m-1, an organic matter content of 6.1 % and a cationic exchange capacity of 46.7 cmol·kg-1 (Yáñez-Díaz et al., 2017). Soil water retention (kg H2O·kg-1 soil; average ± standard deviation, n=3) at a field capacity (-0.03 MPa) and permanent wilting point (-1.5 MPa) at a 0-50 cm depth is 0.3055 ± 0.009 and 0.1901 ± 0.0033, respectively, as previously documented by González-Rodríguez et al. (2004). Predominant vegetation in the study area is the Tamaulipan thornscrub (TTS) (González-Medrano, 2004).
Four species were selected, two trees (Cordia boissieri A. DC or Anacahuita (Boraginaceae) and Prosopis laevigata Humb & Bonpl. ex Will M.C. Johnst or Mezquite (Fabaceae)) and two shrubs (Helietta parvifolia A. Gray ex Hemsl Benth or Barreta (Rutaceae) and Karwinskia humboldtiana Schult Zucc or Coyotillo (Rhamnaceae)). Four representative individuals (repetitions) were randomly identified per species, in an established plot of 2,500 m2, without diseases or mechanical damages (Krug, 2017). Water potential measurement (ΨH, MPa) for the four species were realized in 15 days-intervals from August 1st to October 28th of 2016 at 06:00 a.m. (predawn, ΨHam) and at 2:00 p.m. (midday, ΨHmd). These measurements were realized in the field between 10 and 15 seconds, sampling and cutting a terminal twig (<0.5 cm of diameter) (Krug, 2017), with totally expanded leaves from the medium part of the top of each individual. The duration of the determination of water potential for the four species in both times varied from 10 to 20 minutes depending on sampling season. Water potential was determined using a Scholander pressure chamber (Model 3005, SoilMoisture Equipment Corp., Santa Barbara, CA, USA), in which highly pure nitrogen gas (N2) was used as a pressurization source (Ritchie & Hinckley, 1975).
Temperature (°C), relative humidity (%) and precipitation (mm) for each sampling date were obtained from the meteorological station of the experimental area of the Faculty of Forestry Sciences of UANL. Air temperature and relative humidity variables were used to calculate vapor pressure deficit (VPD, kPa) (Rosenberg et al., 1983). For the gravimetric soil water content (%) in each sampling date, soil samples were randomly extracted inside of the established plot at depth of: 0-10, 10-20, 20-30, 30-40 and 40-50 cm; for it, a Veihmeyer type split barrel (Model 215, Soilmoisture Equipment Corp.) was used. Soil samples were placed in properly labelled aluminum melting pot, which were transported in a polystyrene ice bucket to prevent samples from absorbing or losing humidity. Gravimetric soil water content was determined by means of drying soil sample in a forced air oven (16EG, Gravity Convection Oven) at 105 °C for 72 hours until reaching a constant weight (Taylor & Ashcroft, 1972). In each sampling date for each depth, four repetitions were used to determine soil water content. In addition, soil samples were taken to determine physic-chemical properties (AOAC, 1997) at a depth of 0-20 and 20-40 cm, to evaluate apparent density (Mg·m-3) and some chemical properties such as pH, electrical conductivity (µS·cm-1), organic matter content (%), organic carbon (%) and macro and microelements concentration (mg·kg-1 of soil). Soil physical and chemical determinations are presented in Table 1, realized at the beginning of the study, considering four repetitions.
Table 1 Physical and chemical properties of soil determined at 0-20 cm and 20-40 cm depth in the study site.
| Item | Profile depth | |
|---|---|---|
| 0-20 cm | 20-40 cm | |
| Sand (g kg-1) | 250.75 | 91.50 |
| Silt (g kg-1) | 500.00 | 520.00 |
| Clay (g kg-1) | 250.25 | 389.50 |
| Bulk density (Mg m-3) | 0.93 | 1.10 |
| pH (CaCl2; 0.01 M) | 6.75 | 7.02 |
| EC (µS cm-1) | 128.51 | 103.03 |
| Organic matter (%) | 6.79 | 3.22 |
| C (%) | 3.94 | 1.87 |
| N (%) | 0.43 | 0.18 |
| Ca (mg kg-1 dry soil) | 7981.99 | 7811.06 |
| K (mg kg-1 dry soil) | 213.03 | 102.54 |
| Mg (mg kg-1 dry soil) | 271.00 | 134.35 |
| P (mg kg-1 dry soil) | 4.74 | 2.63 |
| Cu (mg kg-1 dry soil) | 0.36 | 0.74 |
| Zn (mg kg-1 dry soil) | 1.36 | 0.58 |
| Fe (mg kg-1 dry soil) | 3.59 | 4.88 |
| Mn (mg kg-1 dry soil) | 10.60 | 26.54 |
To detect significant differences in ΨHam and ΨHmd among species and soil water content at different depths, data were submitted to normality and variance homogeneity tests according to procedures of Kolmogorov-Smirnov, Shapiro-Wilk and Levene (Brown & Forsythe, 1974). Since most of the samplings did not present neither normality nor variance homogeneity, Kruskal-Wallis non parametric test was used (P ≤ 0.05) to detect significant differences among species and for ΨHam and ΨHmd during each sampling date. Data of gravimetric soil water content at different depth were submitted to an analysis of variance under an experimental design with a classification criterion to detect (P ≤ 0.05) significant differences (Ott, 1993). In order to evaluate the relation of ΨHam and ΨHmd with air temperature, relative humidity, precipitation, gravimetric soil water content and vapor pressure deficit, an analysis of Spearman correlation (rs) was realized. All statistic procedures were realized with SPSS statistic software (Statistical Package for the Social Sciences, version 13.0 para Windows. SPSS Inc., IL, USA).
Results and Discussion
The wide variability of environmental conditions which occurred in different dates during the sampling period was mainly due to the climatic heterogeneity typical of a semiarid region of northeastern Mexico (González-Rodríguez et al., 2011a). In this sense, a temperature and relative humidity were higher before predawn (06:00 h) for the most wet sampling date (September 30th) of 20.0 °C and 96 %, respectively, while for the driest sampling date (August 1st), temperature was 23.2 °C and relative humidity 80 % (Figure 1). On the other hand, at midday (2:00 p.m.), during the most wet sampling date, registered temperature and relative humidity were 27.6 °C and 73.0 %, respectively. In turn, in the driest period, a temperature of 38.4 °C and a relative humidity of 30.0 % were registered (Figure 1). Precipitation for the wet sampling date was 385.4 mm, which is above the one reported by González-Rodríguez et al. (2016). These authors documented 295 mm for the most wet sampling date, while the driest sampling date registered only 0.2 mm (Figure 1), which coincide with what González-Rodríguez et al. (2018) reported, whose reported values of 0.4 mm.
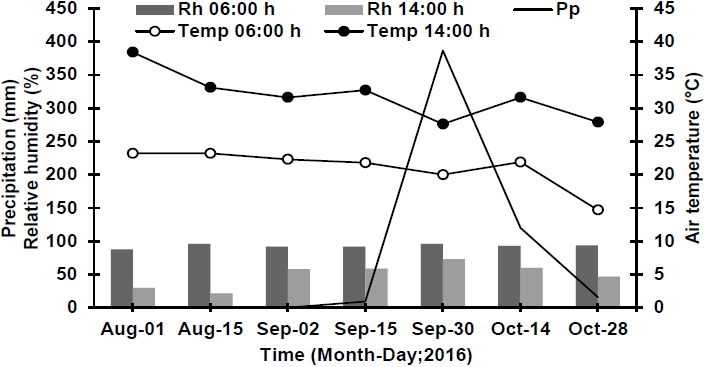
Figure 1 Relative humidity (Rh), air temperature (Temp) at 6:00 h and 14:00 h, and precipitation (Pp) at different sampling dates.
Fort soil water content, from the seven realized sampling dates, only tree (August 15th, September 2nd and September 30th) presented significant differences (p≤ 0.05) among the different depth profiles of the soil (Table 2). The highest values in water content were registered on September 30th (the most wet sampling date), where profiles of 0-10 cm and 10-20 cm reached values of 31.9 and 24.6 %, respectively (Figure 2), being the most responsive to precipitation events. This response was due to the fact that this profile presents a silty-clay-loam soil counting with a high capacity of water storage (Shaxson and Barber, 2005), in combination with a high organic matter content (high carbon and nitrogen contents) and low electrical conductivity (Yáñez-Díaz et al., 2017), which allow water availability for the superficial radicular system of the four studied species, fostering ΨH recuperation. On the contrary, during the driest sampling date (August 1st), profiles of 0-10 cm and 10-20 cm showed a water content of 12 % and 10 %, respectively, which could be influenced by high temperatures, low precipitations and a high evaporative demand (González-Rodríguez et al., 2016), influencing a low water availability in soil for the roots of the studied species, inducing a reduction in ΨH. Water contents are similar to those reported by González-Rodríguez et al. (2018) in studies realized in TTS, where these authors reported values of 10 % and 14 % for previously mentioned profiles, during the driest date, respectively.
Table 2 F and p values from the analysis of variance for soil water content at different soil depts, and χ2 and p values of the Kruskal-Wallis test corresponding to predawn (ΨHam) and midday (ΨHmd) water potential among studied species at different sampling dates.
| Time (Month- Day; 2016)| |
Gravimetric soil water content |
ΨHam | ΨHmd | |||
|---|---|---|---|---|---|---|
| F Value | p Value | χ 2 | p Value | χ2 | p Value | |
| Aug-01 | 0.127 | 0.970 | 9.315 | 0.025 | 3.240 | 0.356 |
| Aug-15 | 32.019 | <0.001 | 10.331 | 0.016 | 5.735 | 0.125 |
| Sep-02 | 3.694 | 0.028 | 8.237 | 0.041 | 2.769 | 0.429 |
| Sep-15 | 0.842 | 0.520 | 14.462 | 0.002 | 6.883 | 0.076 |
| Sep-30 | 54.695 | <0.001 | 2.888 | 0.409 | 2.222 | 0.528 |
| Oct-14 | 1.943 | 0.155 | 6.429 | 0.093 | 9.629 | 0.022 |
| Oct-28 | 0.716 | 0.594 | 15.448 | 0.001 | 12.122 | 0.007 |
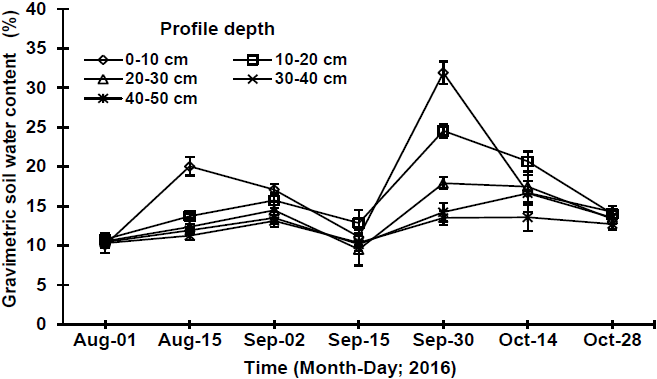
Figure 2 Gravimetric soil water content at five depths (cm) at different sampling dates. Plotted values represent the mean ± standard error of mean (n=4).
Of the seven realized sampling dates, five of them showed significant differences (p≤0.05) in water potential at predawn among the studied species (Table 2). Generally, ΨHam ranged from -1.3 MPa (C. boissieri) to -5.1 MPa (H. parvifolia). During the most wet sampling date (September 30th), maximum ΨHam was of -1.4 MPa (H. parvifolia) and the minimum of -1.8 MPa (P. laevigata) (Figure 3). Regarding the driest sampling date (August 1st), the highest ΨHam (-2.1 MPa) and lowest ΨHam (-5.1 MPa) were detected in P. laevigata and H. parvifolia, respectively (Figure 3).
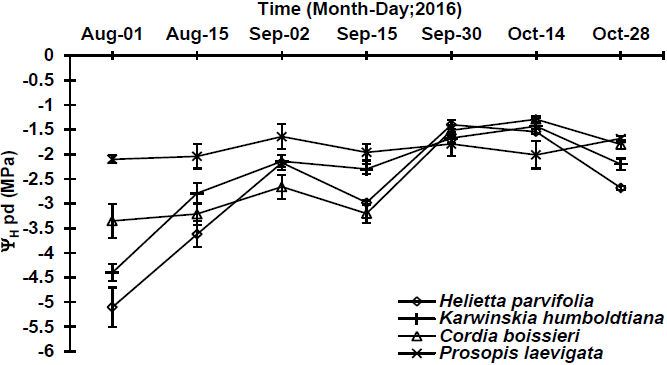
Figure 3 Predawn (ΨHam: 06:00 h) water potential in four native plant species, determined at different sampling dates. Plotted values represent the mean ± standard error of mean (n=5).
Regarding ΨHmd, significant differences (p≤0.05) were detected among the evaluated species in two sampling dates (Table 2). In relation to ΨHmd behavior, values varied from -1.5 MPa (P. laevigata) to -4.0 MPa (K. humboldtiana). During the most wet sampling date (September 30th), ΨHmd varied from -1.7 (H. parvifolia) to -2.1 MPa (C. boissieri) (Figure 4); during the driest sampling date, maximum was -2.9 MPa and minimum -4.6 MPa for P. laevigata and K. humboldtiana, respectively (Figure 4).
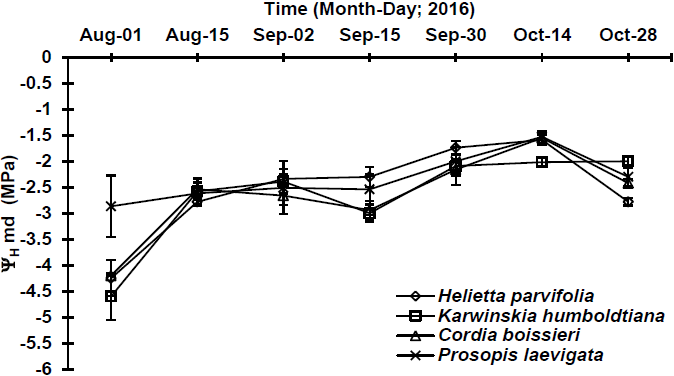
Figure 4 Midday (ΨHmd: 14:00 h) water potential in four native plant species, determined at different sampling dates. Plotted values represent the mean ± standard error of mean (n=5).
Water potentials during the most wet sampling date for H. parvifolia and C. boissieri were low in relation with those observed by González-Rodríguez et al. (2001) in the same species established in a plantation, which could explain that this difference of ΨH, since there was no competence for water resources among more species (Meinzer et al., 2014). ΨH during drought period indicate that H. parvifolia, K. humboldtiana and C. boissieri were submitted to a water stress, behaving as anisohydric species (Meinzer et al., 2014), avoiding, maybe, the stomatal closure, showing very low water potential in conditions of limited water availability in soil, high value of vapor pressure deficit and low relative humidity in the environment (Urli et al., 2013). As well, the above described may indicate that the previously mentioned species can show a superficial root system that prevents absorbing water from profiles of deep soils (Shao et al., 2008). In this regard, with a low water content in soil, the lowest ΨH (-5.1 MPa) was present in H. parvifolia, since its root system is superficial, reaching depth no higher than 40 cm (González-Rodríguez & Cantú-Silva, 2001); in turn, the highest ΨH (-2.1 MPa) was present in P. laevigata, since this species presents a deep taproot system, that can reach until 80 m depth, possibly allowing it to absorb water from deeper soil profiles (Rodríguez-Sauceda et al., 2014).
ΨH at predawn showed differences among species and sampling dates (wet and drier). In this sense, ΨH ranged from -1.3 MPa to -5.1 MPa, respectively. These values were found below those observed by González-Rodríguez et al. (2016) in a study of water potentials in native plants from northeastern Mexico, presenting values that varied from -0.30 MPa to -3.94 MPa in the most wettest and driest sampling date, respectively. For the information above explained, it can be inferred that, during the study realized by these authors, environmental conditions were less stressing for the studied species since they present a lower water stress. However, results obtained during the present study were similar to those obtained by González-Rodríguez et al. (2011a) where potentials fluctuate from -0.7 MPa to -4.5 MPa. Similar results to the values of potentials for the previously mentioned species have been observed similarly by González-Rodríguez et al. (2011b), in tree species such as Acacia amentacea Benth. (Leguminosae, shrub with deciduous leaves; microphyllous compounds leaves), Celtis pallida Torr. (Ulmaceae; thorny shrub with perennial oval-shaped leaves with smooth edges), Forestiera angustifolia Torr. (Oleaceae, shrub with perennial leaves and rigid and dense branches) and Parkinsonia texana (A. Gray) S. Watson var. Macra. In some sampling dates, ΨHmd were detected to be higher than ΨHam (Figures 3 and 4). This behavior was observed in H. parvifolia, K. humboldtiana, C. boissieri (August 15th) and in P. laevigata (October 14th). This is related to the stomatal closure of the leaves to avoid water loss by transpiration (Meinzer et al., 2014), or to an imbalance between soil water potential at predawn and in plant tissue (leaves and xylem) caused by solute accumulation (Donovan et al., 2003), which are typical mechanisms that plants have to face at low soil water contents and extreme environmental conditions (Meinzer et al., 2014). Similar results have been described by González-Rodríguez et al. (2016), who reported a similar behavior in Celtis ehrenbergiana, Acacia amentacea, Parkinsonia texana and Forestiera angustifolia.
Derived from the Spearman correlation analysis (rs) (n = 28), for ΨHam and ΨHmd with environmental variables, ΨHam showed a significantly positive correlation with soil water content at four depths (10-20, 20-30, 30-40 and 40-50 cm) and with precipitation (Table 3). In the case of ΨHmd, a significantly positive correlation was observed with soil water content at the five depths (0-10, 10-20, 20-30, 30-40 and 40-50 cm). In turn, air temperature and vapor pressure deficit showed a significantly negative correlation and a significantly positive correlation with relative humidity. Finally, ΨHmd showed a negative correlation with precipitation (Table 3). Only ΨHmd of the studied species was strongly influenced by vapor pressure deficit which values ranged from 1.0 kPa to 4.7 kPa indicating that, for this measurement point, vapor pressure deficit influences the variation of water potentials in the studied species. The obtained results differ to what observed by González-Rodríguez et al. (2011a, 2016) in species from northeastern Mexico such as Acacia berlandieri, Acacia rigidula, Eysenhartia texana, Diospyros texana, Randia rhagocarpa, Bernardia myricaefolia, Bumelia celastrina and Condalia hookeri, where a significantly negative relation is reported as ΨHam as for ΨHmd with air temperature and vapor pressure deficit.
Table 3 Spearman correlation coefficients (n=28) for predawn (ΨHam) and midday (ΨHmd) water potential in relation with prevailing environmental conditions.
| Variable | Correlation |
|---|---|
| Water potential before sunrise ΨHam | |
| Gravimetric soil water content | |
| Depth of 0-10 cm | 0.418ns |
| Depth of 10-20 cm | 0.770*** |
| Depth of 20-30 cm | 0.729*** |
| Depth of 30-40 cm | 0.748*** |
| Depth of 40-50 cm | 0.774*** |
| Air temperature | -0.506ns |
| Relative humidity | 0.367ns |
| Vapor pressure deficit | -0.281ns |
| Precipitation | 0.711*** |
| Water potential at noon ΨHmd | |
| Gravimetric soil moisturewater content | |
| Depth of 0-10 cm | 0.498** |
| Depth of 10-20 cm | 0.826*** |
| Depth of 20-30 cm | 0.764*** |
| Depth of 30-40 cm | 0.819*** |
| Depth of 40-50 cm | 0.860*** |
| Air temperature | -0.708*** |
| Relative humidity | 0.670*** |
| Vapor pressure deficit | -0.820*** |
| Precipitation | -0.781*** |
NS=not significant p≥0.05; *p<0.05, **p<0.01; ***p<0.001.
According to ΨH of the four studied species, P. laevigata is an example of a tree species which has adapted to the low water availability, which evidence was that it presents high potentials during the different sampling dates. Therefore, it can be argued that this species, as some other species, presents a reduced leaf area and a high stomatal sensitivity, provoking the closure of its stoma, which are considered as drought evasion strategies allowing to reduce water loss to avoid or reduce tissue desiccation (Jaleel et al., 2009; Luna-Flores et al., 2012); in this way, P. laevigata can be considered as an isohydric species, able to tolerate water deficits, while adaptation of H. parvifolia, K. humboldtiana and C. boisiseri species to an adverse environment, apparently depends on similar strategies allowing them to face tissue desiccation (Fleta-Soriano & Munné-Bosch, 2016), which could have indicated that water potentials of these species were lower. Finally, it is important to notice that this type of research are necessary, since cases of forest death have been reported due to extreme climatic changes in the environment (Urli et al., 2013), it is therefore necessary to consider the background of species able to resist or tolerate adverse conditions as extreme drought allowing them to grow, survive and reproduce to maintain vegetation cover in dry and semiarid regions, and to maintain ecosystemic services.
Conclusions
During the driest sampling date, H. parvifolia, K. humboldtiana and C. boissieri presented minor values in their water potentials, indicating that these species were under an adverse water stress, presenting physiological disadvantages in drought period, which can induce, at a great extent, the absence of survival of individuals from these species, affecting its establishment in the Tamaulipan thornscrub.
High soil water content, as well as environmental variables during the different sampling dates, were responsible for ΨHam and ΨHmd increase in H. parvifolia, K. humboldtiana and C. boissieri, indicating that these three species are dependent of high soil water contents. With these environmental conditions, these species can be used in activities of urban dasonomy with the help of auxiliary water irrigation, which will allow them to maintain their hydration, favoring their growth and development.
Prosopis laevigata seems to be a more resistant species to drought, since its potential at predawn as well as at midday, did not significantly vary in each sampling date, maintaining its potential stable in relation with prevailing environmental variables. This species can be used in activities of reforestation, restoration, habitat for wild fauna or holistic management of grasslands in the management of extensive or intensive cattle raising in dry or semiarid regions.











 texto en
texto en 


