Introduction
The production of citrus in Mexico, especially ‘Persian’ lime (Citrus latifolia Tanaka) focused on fresh consumption and it is leading worldwide exports of fruits (Schwentesius et al., 2005).
The national production in Mexico in 2016 was of 5.75 million tons with a value of $16,781 million Mexican pesos, for this year the planted surface area of different citrus was 533,749 ha, of which 180,252 ha were for Mexican lemon (Citrus aurantifolia (Christm) Swing), 178,877 ha for grapefruit, (Citrus paradisi Macf.) and 335,610 ha for ‘Valencia’ orange (Citrus sinensis (L.) Osbeck) (SIAP, 2017).
In the state of Morelos, Mexico, in 2010 there were orchards of citruses established in 28 of the 33 municipalities, with a surface planted of 1000 ha, with high production and quality of exportation. Nowadays, there is an established surface of 612 ha, in 17 municipalities, where lemon and orange ‘Valencia’ stand out, with 32 and 66 % of the total surface respectively, and a yield of 12.11 and 7.76 t ha-1 respectively, both species being of uttermost economic importance in the state of Morelos, the rest of the surface is composed by other citruses (SIAP, 2017, CESVMOR, 2018). The generalized presence of ‘Persian’ lime trees with symptoms of gummosis, canker, dieback of branches and death of trees has caused reduction in productivity of the orchards and the increasing production costs. The technical recommendations in the state are centered on the use of specific fungicides for the control of several species of Phytophthora, causal agent of gummosis in citruses in other states of Mexico (Acosta-Pérez et al., 2012). However, the results are not satisfactory. The symptoms observed in ‘Persian’ lime trees coincide with those caused by Lasiodiplodia spp in various species of trees (Abdollahzadeh et al., 2010). The first report of the phytopathogen was made by Álvarez (1976) in cacao crop (Theobroma cacao). Recent reports indicate that this pathogen has been isolated from diverse citruses species, in California, USA, such as the ‘Eureka’ lemon, ‘Valencia’ orange, ‘Washington Navel’ orange, navel ‘Fukomoto’ orange, grapefruit, ‘Satsuma’ tangerine and ‘Meyer’ lemon (Adesemoye et al., 2014). It has also been reported that species of Lasiodiplodia are common in the tropics, causing diseases in plants, in pre and post-harvest and that their cellulolytic activity allows for penetration and colonization of the plant in a similar way to those of a soft rot causing-fungus, using the starch and other saccharides present in the initial substrate of the wood (Muñoz et al., 2015). The imports of other fruit hosts of this pathogen from other countries such as the nut and the English walnut (Li et al., 1995), grape (Linaldeddu et al., 2015; Rodríguez et al., 2015), sweet orange, oak, retama: Fabaceae (Linaldeddu et al., 2015), bitter orange (Alves et al., 2008), coconut (Dugan et al., 2015), mango (Johnson et al., 1992; Marques et al., 2013), papaya (Netto et al., 2014), poplar, willow (Hashemi & Mohammadi, 2016) Brazilian firetree (Schizolobium parahyba var. Amazonicum) (Mehl et al., 2014), eggplant (Solanum melongena) (Woodward et al., 2005), loquat (Eriobotrya japonica) (González et al., 2017), jackfruit (Artocarpus heterophyllus) (Ni et al., 2008), golden cane palm (Dypsis lutescens) (Pereira et al., 2015), kenaf (Norhayati et al., 2016), Jatropha (Machado et al., 2014) and various introduced species of forest trees from other countries have eased the dispersion of this pathogen (Segura-Contreras et al., 2015).
Presently, there are reports in Mexico of a great variety of crops throughout the country such as: hibiscus (Hibiscus sabdariffa L.) in Guerrero (Aparicio et al., 2016), papaya (Carica papaya L.) in the central coast of the state of Veracruz (Becerra et al., 2014), heliconia in Tabasco (Ortiz et al., 2014), seeds of Jatropha curcas from collections of Puebla, Veracruz and Chiapas (Nolasco-Guzmán et al., 2014), Aloe vera in Tamaulipas (Zúñiga-Estrada & Yáñez, 2016), avocado (Persea americana), mango (Mangifera indica), coconut (Cocos nucifera), cassava (Manihot esculenta), sugarcane (Sacharum officinarum), citruses (Citrus spp.), Arabian coffee (Coffea arabica), purple yam (Dioscorea alata), sweet potato (Ipomea batata), maize (Zea mays), plum (Prunnus domestica), sisal (Agave sisalana), Caribbean pine (Pinus caribaeae) of the main producing zones (Agroecología, 2018; Picos-Muñoz et al., 2015).
The impact of the fungal species of the family Botryosphaeriaceae on citruses has not been widely explored, since it is probable that the diversity of fungi of this family in citruses is not well known and besides, is causing more damage than it was previously thought (Adesemoye et al., 2014).
The majority of the descriptions of this phytopathogen have been based on their morphological characters and since the last decade, the identification has been complemented with molecular biology techniques (Picos-Muños et al., 2015).
According to studies performed by Phillips et al. (2013), due to the cosmopolitan presence, the wide number of hosts and the morphological variability of this pathogen, there are various cryptic species affecting citrus plantations in Mexico, which are generally separated by size and shape of the conidium. In this sense, the objective of this work was to isolate, identify and molecularly diagnose, the causal agent of dieback on branches and gummosis on plantations of ‘Persian’ lime in the state of Morelos, Mexico.
Material and Methods
Study location
The study was carried out in August 2015, in the state of Morelos, Mexico, geographically located at 22°51′43″ NL and 102°36′38″ WL and at 2,309 m.a.m.s.l., in commercial orchards of ‘Persian’ lime.
The evaluated plantations were located in the municipalities of Tlaltizapan, Ayala, Coatlan del Rio and Amayuca and were selected from the list of producers of ‘Persian’ lime of the Morelos State Plant Health Committe.
Biological material sampling
Plantation sampling was performed according to what Acosta-Pérez et al., (2012) proposed, forty-six samples were collected, from primary and secondary branches, and trunks from trees of five to eight years old, having symptoms of exuding of gum, cankers and dieback on branches, which were transported in polyethylene bags and conserved at 6 °C, until they were processing at the laboratory of Phytopathology of the Escuela de Estudios Superiores de Xalostoc of the UAEM, Xalostoc, Morelos, Mexico (EESuX-UAEM).
Fungus isolation
Samples were selected in fractions of 0.5 cm2, including the area of disease progression. The fragments were disinfested by immersion in a solution of sodium hypochlorite (NaClO) at 2 % for 2 min and three washings with sterile distilled water for 2 min each; then, they were dried in sterile absorbent paper to eliminate the excess of water.
Subsequently, four fragments of tissue per Petri dish, containing potato dextrose agar (PDA) culture medium were incubated under white light at 24 °C ± for three days. Once the growth of the fungus occurred, the colonies were purified by means of the technique of hypha tip, in PDA culture medium to increase the colony.
The monosporic isolates were re-sown in vegetable juice V8 juice agar culture medium (Ortiz, 1996), then, the isolates were stored in test tubes with culture medium PDA covered in oil (Crous et al., 2006).
Morphological identification and characterization of the isolates
The identification of the isolated was performed using taxonomic keys and descriptions by Phillips et al., (2013), based on the morphological characters of the ascospores, conidial states, pigmentation, thickness of the wall and shape of the conidia, dimensions, septation, presence of paraphyse in the pycnidia and striations. The observation was carried out with a light field stereoscope Olympus®.
Pathogenicity tests
In order to verify the pathogenicity of the fungus, Koch’s postulates and the technique described by Agrios (2005) were performed.
Certified one-year-old ‘Persian’ lime were inoculated, at a height of 15 cm from the ground, the stems were disinfected with sodium hypochlorite (NaClO) at 1.5 %. Then, an incision was made on the bark of the stem with a scalpel, of approximately 7 mm. Later on, discs of 0.5 cm of the isolate of the fungus were inoculated. The inoculation was performed by means of placement of the mycelium with culture medium in the openings of the stems, the control only received culture medium without the plant pathogen. In order to favor the infection, the inoculated area was covered with humidified cotton with sterile distilled water and covered with Parafilm®. The plants were kept individually under open field conditions. Visual evaluations were performed ever 15 d for three months, recording the evolution of the symptoms of the disease on the inoculated plants.
When detecting the symptoms of the disease, the next step was re-isolation, with the purpose of verifying that the inoculated fungus corresponds originally to the isolated one. The morphological characteristics of the inoculated fungus were compared, in addition to the symptoms that the inoculated plants presented, according to the incubation period and damage of the tissue.
Molecular identification
The molecular identification was conduced at the laboratory of Phytopathology of the Centro Nacional de Investigación Disciplinaria en Conservación y Mejoramiento de Ecosistemas Forestales, (INIFAP) Coyoacán, Mexico City, Mexico.
The extraction of genomic DNA (gDNA) was carried out using the AP method described by Sambrook & Russell (2012), using the mycelium of the monosporic culture of the obtained isolates. The quality was assessed by horizontal electrophoresis in agarose gel at 1 % (Ultrapure, Gibco, USA) and the bands were visualized in a transilluminator (Gel Doc 2000, BIO RAD®, USA). The concentration of DNA was quantified with a spectrophotometer NanoDrop 2000 (Thermo Scientific®).
The primers used for the molecular identification of the fungi were ITS5 (5’-TCC TCC GCT TAT TGA TAT GC-3’) e ITS4 (5’-GGA AGT AAA AGT CGT AAC AAG G-3’) of the ribosomal gens (rDNA).
The analysis of the samples was corroborated by means of PCR according to the protocol described by Ahrens and Seemüller (1992), with modifications on the components such as: sterile ultrapure water (16.25 μL), TBE IX buffer solution (2.5 μL), MgCl2 at 2.5 mM (1.25 μL), dNTPs at 0.2 mM (0.5 μL), ITS5 and ITS4 universal initiators at 20 mol (1 μL of each), DNA polymerase (Promega®) at 1U (0.5 μL) and 80 ng (2 μL) of gDNA previously extracted according to Sambrook and Russell (2012).
Phylogenetic analysis
DNA fragments amplified by PCR were purified using the Wizard kit (Promega®, USA), following the protocol recommended by the manufacturer. PCR purified product was sequenced by Macrogen Inc. (Seoul, South Korea).
The sequences were assembled, and edited with the CAP option (Contig Assembly Program) of the BioEdit v7.0.9.1 Software (Hall, 1999). The sequences of fungi were compared and deposited in GenBank database (NCBI, 2016).
The phylogenetic trees were constructed with the data from ITS5 and ITS4 terminations; additionally they were analyzed with the MEGA software (Molecular Evolutionary Genetics Analysis) version 7.0.14 (Swofford, 2003; Tamura et al., 2007), aligned with ClustalW (Thomson et al., 1994) and compared with the sequences of homologous gens deposited on NCBI database (National Center for Biotechnology Information, NCBI, 2016).
After analyzing the congruence among data sets, a maximum parsimony (MP) analysis, the phylogenetic analyses were performed with PAUP (Phylogenetic Analysis Using Parsimony) version 4.0b10 (Swofford, 2002). Phylogenetic trees were obtained with the heuristic search function with 1,000 repetitions of randomized addition, tree bisection and reconstruction (TBR) as algorithm of exchange of branches and the spaces or missing data were considered as complete deletions. The levels of significance of the tree ramification points were determined by means of bootstrapping with 1,000 repetitions (Kimura, 1980; Felsenstein, 1985; Hillis & Bull, 1993). The HG231345 sequence of Phoma tracheiphila from GenBank was used as the taxon out of group.
Results and Discussion
Morphological identification and characterization of the isolates
From the 46 sampled trees of ‘Persian’ lime, 16 isolates were obtained, presenting symptoms in primary and secondary branches with dieback and gummatous exudates. Only three of them were morphologically identified according to the taxonomic keys reported by Phillips et al., (2013).
In the observation by stereoscopic microscope, colonies with growth of smoked gray mycelium were identified, having from olive gray to greenish gray to dark blue slate and the presence of pycnidias 30 d after incubation. Just like conidia produced and formed by one single cell, oval-shaped, hyaline and double-wall with 17- 26 X 12 µM in size in immature colonies. In mature colonies, the conidia showed a dark brown coloration, two cells and striations and 18-28 X 11-16 µM in size, been similar with asexual reproductive structures of the genus Lasiodiplodia, verruculous wall <2 µM, 1-septun with longitudinal striations, which agrees with that reported by Phillips et al., (2013).
Similar results were obtained by Slippers et al. (2004) and Alves et al. (2008), achieving moderately dense colonies in culture medium, with aerial mycelium that was initially white and the turning olive-gray 7 d later, and finally obtaining a dark blue slate.
Damn et al. (207) and Netto et al. (2014) confirmed that the main characteristic distinguishing Lasiodiplodia genus from other closely related genera is the presence of pycnidia, paraphyses and longitudinal striations in mature conidia, which coincides with what was observed in this study.
The dimensions of the conidia and the paraphyses, agree with what was established by Pitt & Hocking (2009), Phillips et al. (2013) and Muñoz et al. (2015) for this genus.
Varela et al. (2013) described them as the causal agent of death of bitter orange plants engrafted with different species of citruses in nurseries, as well as the death of branches in Mexican lemon trees and of rot of the peduncle in orange fruits.
The confusion on the diagnostic of the pathogen agent is because it produces very similar symptoms as tose by Phytophthora parasitica, like the formation of gummosis, leaf spots, fruit rotting, dieback, perennial cankers and death of trees, all of these are associated with several species of fungi of Botryosphaeriaceae (Muñoz et al., 2015).
It has to be pointed out that up to now, this disease had not been reported for the state of Morelos on ‘Persian’ lime, reasons that would justify the inefficiency of its management in the field.
Three species of the Lasiodiplodia genus were morphologically identified and characterized. The first one showed colonies with absent conidiophores, holoblastic conidiogenic cells are discreet, hyaline, flat, of thin-wall, cylindrical, proliferating with 1-2 rings, 11-16 X 3-5 µM. Conidium initially hyaline, aseptic, ellipsoid to ovoid, with granular content, both ends widely rounded, wall <2 µM, pigmented, verruculous, ovoid, 1-septated with longitudinal striations, corresponding to L. citricola species (Figure 1).

Figure 1 Branch in humid chamber (A), isolated strain (5 d) (B), formation of pycnidia after 7 days in humid chamber (C), body of the picnid and conidio of L. citricola, (D) picnidio cut where paraphyses, growing and immature conidia are observed (E).
Another species identified and characterized was L. pseudotheobromae, with ellipsoidal conidia, rounded apexes and bases, wider in its center at two superior thirds, of thick walls, hyaline, and with no septa.
The third species corresponded to L. theobromae (Pat.) Griffon and Maubl., presenting hyaline conidiogenic cells, whit thin wall, flat, cylindrical to sub-pear-shaped, holoblastic, discreet, determined or undetermined and proliferating by one or two distinct rings, or proliferating at the same level, making room for periclinal thickening, the conidium is sub-ovoid to ovoid ellipsoid, widely rounded apex, sharpening at the truncated base, wider in the medium third to superior third, thick walls, granular content, initially hyaline and accepted, staying hyaline, coming to be dark brown and only 1-septate. Then, it produces the unloading of conidioms, with melanin deposits on the interior surface of the longitudinally disposed wall, giving a striated appearance to the conidia.
L. theobromae, is cosmopolitan and has a wide range of hosts (approximately 500), including monocotyledons, dicotyledons, and gymnosperms, is a pleomorphic and ubiquitous fungus, which has produced more than one synonym (Abdollahzadeh et al., 2010; Wang et al., 2011, cited by Picos et al., 2015). Although it has a wide geographical distribution, it is common in the tropics and subtropics This non-specialized plant pathogen has been reported causing numerous diseases, including dieback, root rot, fruit rot, leaf spots and “witch’s broom”, among many others. It can also be an endophyte (Mohali et al., 2005). Given that its occurrence is generalized, their great quantity of hosts and their known morphology and variability, it is possible that L. theobromae is composed of a quantity of cryptic species (Punithalingam, 1980).
Lasiodiodiplodia pseudotheobromae is a species related to L. theobromae and has been recently described in Acacia, Citrus aurantium, Coffea, Gmelina and Rosa (Alves et al., 2008). It has been reported in different species of Citrus, but up to now it had not been reported in ‘Persian’ lime.Slippers & Wingfield (2007), reported these phytopathogens as endophyte species in commercial orchards of citruses in Mexico.
Pathogenicity tests
The pathogenicity tests performed on certified ‘Persian’ lime plants, revealed that the isolated fungi in this study were pathogens for this culture, observing the first symptoms at 22 DAI (days after inoculation), thus confirming that the identified species of the Lasiodiplodia genus were associated wiht this disease and were able to cause chlorosis on leaves, dieback on primary and secondary branches, formation of canker and gummatous exudates.
The leaves of sick trees turned chlorotic, compared to the control’s leaves. This symptomatology became more notorious on some plants, in addition the stems showed brownish wounds. In inoculated plants, damages characteristic on stems were not observed.
At 29 DAI, the plants showed leaves with marked chlorosis in the central nerve, while healthy plants did not show damage on their stems and leaves.
At 36 DAI, a marked chlorosis with respect to the other observations was noted, finding yellow leaves, but a little rolled, and formation of a wound in the base of the stem (rot) of some plants.
At 42 DAI, some trees showed the formation of a well-defined canker at the base of the stem. The control trees (not inoculated), did not show symptoms of any disease and kept on being healthy until the end of the evaluations. Moriwaki et al., (2003) reported that the pathogenicity tests and morphological characters allow to taxonomically separate the species of the Lasiodiplodia genus. These results demonstrate the importance and current validity of the traditional tools for separating the species in the taxonomy of fungi. Also, very important for producers of ‘Persian’ lime, who export a large part this fruit. The molecular and morphological characters showed the same species of inoculated Lasiodiplodia.
Molecular identification
Studies performed by PCR using ITS5/ITS4 primers showed the amplification of DNA fragments of 500 pb starting from the fungal mycelium of the samples for all the isolates.
The sequence analysis showed that the fungi correspond to Lasiodiplodia citricola, L. pseudotheobromae and L. theobromae. However, 13 of the obtained isolates, could not be aligned to any species of the Lasiodiplodia genus.
These results suggest that these pathogen species prevailing in the orchards of citruses constitute a threat to plantations health (Timmer et al., 2002), then adequate control treatments have to be implemented, since this disease is going to be an important economic problem in the region, due to the high susceptibility of ‘Persian’ lime to this disease.
Bautista-Cruz et al. (2018) studied on the phylogeny, distribution and pathogenicity of Lasiodiplodia species associated to cankers and symptoms of dieback in ‘Persian’ lime in the states of Veracruz and Puebla, Mexico, where Lasiodiplodia pseudotheobromae and L. theobromae were isolated, which are similar with the present study, suggesting that both species are spreading throughout the citrus plantations in Mexico, constituting a threat for national production.
Guajardo et al. (2018). reported L. theobromae for the first time in Chile causing gummosis in lemon. Then, it is why constant monitoring of this pathogen is important so that it does not extend its dissemination in the main commercial orchards or citruses in Mexico.
Adesemoye et al. (2014) reported other species of Botryosphaeriaceae in ‘Persian’ lime in California, United States of America, such as Diplodia seriata and Dothiorella viticola.
Other studies on genetic diversity affirm that the two cryptic species of Lasiodiplodia (L. theobromae and L. pseudotheobromae) have not been found in the same host, suggesting that a possible hybridization between them have been found yet (Begoude et al., 2010). On the other hand, Al-Sadi et al. (2013) found a moderate level of genetic diversity in populations of three species of Lasiodiplodia from different hosts and geographic origins, and a high number of not-specified genotypes.
The molecular markers have recently been used for examining gens and genotypes, modes of reproduction and speciation of a number of fungi, including Botyosphaeria spp. and their anaphorms (Burgess et al., 2006).
The 15 obtained sequences were deposited in Genbank with access numbers for: Lasiodiplodia citricola (KY271187), Lasiodiplodia pseudotheobromae (KY284596), Lasiodiplodia theobromae (KY284589, KY284587) and Lasiodiplodia spp. (KY284584, KY284595, KY284594, KY290890, KY284592, KY284593, KY284588, KY284591, KY284586, KY284585 and KY284590), respectively.
Phylogenetic analysis
The phylogenetic tree obtained from the DNA analysis of the 16 isolations of Lasiodiplodia is shown in Figure 2.
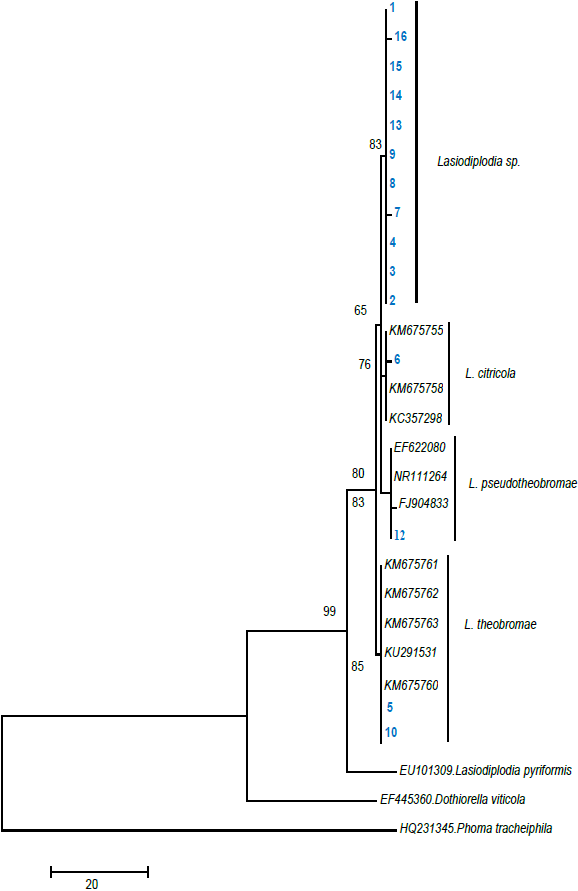
Figure 2 Phylogenetic tree based on Bayesian and maximum parsimony analysis using ITS 5 and ITS 4 primers.
Species groups did not change, maintaining a similar topology of the cladograms. In the bayesian analysis, the position of the clades (groupings) of Lasiodiplodia among each other were different, but inside of each species the topology was similar to the tree with maximum parsimony (TreeBase 11035). In this study, the following four clades were identified: Clade 1 (Lasiodiplodia spp.), Clade 2 (L. citricola), Clade 3 (L. pseudotheobromae) and Clade 4 (L. theobromae). The species Dothiorella viticola (EF445360) and Phoma tracheiphila (HQ231345) were considered out of the group.
Different studies consider that a support high or equal to 70 % provides reliable data about the positioning of each species in the cladogram (Arnold et al., 2007), which agrees with the results obtained in the present study.
As a consequence, the formation of the tree allowed the conformation of groupings, which confirm the presence for the first time of three species of the Lasiodiplodia affecting the plantations of ‘Persian’ lime in the state of Morelos, Mexico.
Conclusion
The symptoms observed in plantations of ‘Persian’ lime, with dieback and gummatous exudates, from the municipalities of: Tlaltizapan, Ayala, Coatlan del Rio and Amayuca, in the state of Morelos, 16 isolates were obtained, from which three species were identified corresponding to Lasiodiplodia as L. citricola, L. pseudotheobromae and L. theobromae. Also, it was confirmed for their morphology, pathogenicity and molecular analysis performed on the ITS 5 and ITS 4 gens, with a genetic distance up to 99 %, which were grouped into four clades according to the phylogenetic tree and located in GenBank. This is a new reports of fungal pathogenic agents in ‘Persian’ lime for the state of Morelos, Mexico.











 texto en
texto en 


