Introduction
Mango is a product that plays an important socio-economic role both at national and world level in different countries, basically in developing countries. It is the third tropical fruit worldwide, considering production and import (after banana and pineapple) and fifth of all the fruits commercialized globally. For Mexico, mango is also a very important source of employment, income and generation of foreign currency (COFUPRO, 2003).
The food and beverage industry is one of the largest industrial sectors in the world and of vital importance for economy. Producing sub-products in this sector is an economic and environmental problem steadily increasing. Although the food industry does not produce waste as dangerous as other sectors, it generates a high volume of biodegradable waste, causing water contamination, damage to vegetation and greenhouse gas emissions (Zamorano et al., 2007). In the case of commercialization and transformation of tropical and subtropical fruit, specifically mango, its consumption fresh has expanded at world level because of its excellent sensory (color, sweetness and aroma) properties and nutritional (vitamin, mineral, fiber and phytochemical) composition (Kim et al., 2009).
Parallel to fresh mango marketing, the demand for processed mango is increasing globally. Mango industrialization is a form of decreasing losses in high season and maximizing its exploitation. Approximately 20 % of the fruit is processed into products, such as purée, nectar, juice, pickled and canned slices, sweet and spiced jam, spicy sauce and candy, dessert flavoring, among others; these products have become more and more popular all over the world and gained importance in American and European markets (Ribeiro & Schieber, 2010).
In the food industry, after mango processing or transformation, sub-products, which are not used most of the time, are generated and discarded as waste, considering them a serious contamination problem due to the production of methane and other decomposing materials (Ajila et al., 2010).
Several research studies on proximal composition of mango seed have shown it is comparable to the majority of the cereals: carbon hydrates (69.2 %-79.8 %), fat (8.3 %-16.1 %), protein (5.6 %-9.5 %), fiber (0.1 %-2.9 %) and ashes (0.3 % a 3.6 %) (Legesse & Emire, 2012). Soong et al., (2004) reported that the mango seed embryo shows an important antioxidant activity due to its high content of phenolic compounds, and it can be a good source of phytosterols and tocopherols. Schieber et al. (2003)&Núnez-Selles (2005), reported that the antioxidant activity of the embryo is due to its high content of polyphenols, terpenoids, phytosterols and microelements, such as selenium, copper and zinc.
Studies in mango skin have pointed it out as a potential source of phytochemicals: polyphenols, carotenoids, enzymes, vitamin E and vitamin C (Ajila et al., 2007a), besides showing antioxidant properties (Ajila et al., 2007b). According to Masibo and He (2009), the proximal composition of green and mature mango skin of two varieties (Badami & Raspuri) is the following: humidity 70.5 ± 4.5 (greater in mature mango skin), total protein 1.90 ± 0.15, lipids 2.41 ± 0.25, carbohydrates 24.0 ± 4.0, crude fiber 5.35 ± 2.05 (greater in mature mango skin) and ashes 2.08 ± 0.92. While Alija et al. (2007a) found the following fiber percentages in mango skin: total dietary fiber 45-78 %, soluble dietary fiber 16-28 % and insoluble dietary fiber 29-50 %.
In the case of industrial agricultural mango sub-products, an important concentration of bioactive compounds has been reported, particularly mangiferin, a xanthone glucoside that shows antioxidant activity (Shah et al., 2010). More nutritional and pharmaceutical potential needs to be explored from mango sub-products since skin, seed and pulp, which are not used, are favorable raw materials to obtain rich extracts in phenolic compounds with antioxidant properties. Moreover, their exploitation could reduce environmental problems caused by this waste. (bdeldaiem & Hoda, 2012; Kittiphoom, 2012; Padmaja & Prasad, 2012; Dorta et al., 2012; Somkuwar & Kamble, 2013; García-Magaña et al., 2013;)obtained after processing, contain large amounts of compounds with antioxidant activity that can be reused to reduce their environmental impact. The present study evaluates the effect of solvent (methanol, ethanol, acetone, water, methanol:water [1:1], ethanol:water [1:1], and acetone:water [1:1].
Therefore, the objective of this research study was to assess the antioxidant activity and concentration of some bioactive compounds of pulping sub-products of three mango cultivars.
Material and Methods
The units of study were mango (Mangífera indica L.) fruit from Ataulfo, Kent and Keit, harvested during July 2012 in the municipality of Tepic (Latitude: 21.5039, Longitude: -104.895, 21° 30′ 14″ N, 104° 53′ 42″ W) in the state of Nayarit, Mexico. Fruit harvest was performed manually at consumption maturity and transported in wood boxes to the laboratory where they were stored for three days in refrigeration at 4°C in a cold chamber in complete darkness until its processing and analysis.
Sample preparation
Mango fruit (n = 35 mangoes) were taken from each one of the three cultivars. They were washed with soap and tap water, weighed and processed in a pulping experimental type machine (Veyco); afterwards the sub-products obtained from pulping (skin, seed and pulp adhered to both) were recollected separately depending on the cultivar and crushed in a cereal mill to obtain a paste; 100 g of paste from each cultivar were lyophilized until constant weight was obtained. Then, an ethanolic extraction was performed for three hours, and the supernatant was sieved through Whatman #4 (Maisuthisakul & Gordon, 2009) filter paper; the extracts obtained were stored in refrigeration at 4°C until analyses were performed in periods no greater than eight days. Sample storage was made in opaque containers in darkness both in a cold chamber as in the refrigerator. Only was the lyophilized sample used and not the ethanolic extract for the carotenoid and tocopherol analyses.
Determination of bioactive compound concentration of mango paste sub-product
Determination of total phenolic compound concentration
Total phenolic compounds were determined according to the method of Stintzing et al. (2005) that consisted of placing 100 µL of sample in 1.5-mL microtubes, to which 500 µL of Folin-Ciocalteu (1:10 in distilled water) and 400 µL sodium carbonate (at 7.5 %) solutions were added; then, the samples were agitated in vortex and incubated at room temperature for 30 min. After time elapsed, absorbance was measured at a wave length of 765 nm. Total phenolic compound concentration was obtained starting from a standard curve of gallic acid from 0 to 400 mg/L. The results were expressed in milligrams Equivalent to Gallic Acid (EAG)/g of paste in dry weight.
Determination of total flavonoid concentration
Total flavonoid concentration was determined according to the method described by Zhishen et al. (1999) with slight modifications, taking 50 µL of each sample, mixing with 100 µL distilled water, then adding 10 µL NaNO2 (15 %) solution and agitating in vortex. After letting set for six minutes at room temperature, 15 µL of AlCl3 (10 %) solution were added and left to settle again for six minutes; next, 200 µL of NaOH (4 %) solution were added to the mixture and agitated in vortex. Mixture absorbance was determined at 510 nm. The results were expressed as mg equivalent to quercetin per gram of paste in dry weight. The standard curve was performed from 0 to 1 mg of quercetin/mL.
Determination of mangiferin content
The colorimetric method was used as described by Chang et al. (2002), in which 50 µL of each sample were taken and diluted with 150 µL ethanol; 10 µL of AlCl3 (10 %) and 300 µL of potassium acetate (0.03 M) were added; immediately after, the sample was agitated in vortex and incubated at 30°C for one hour . Later, absorbance of the sample was read at a wavelength of 410 nm. The standard curve was performed from 0 to 200 µg mangiferin/mL of DMSO. The results were expressed in micrograms equivalent to mangiferin per gram of paste in dry weight.
Determination of ascorbic acid concentration
To determine ascorbic acid content, the colorimetric method was used as described by Dürüst et al. (1997). The procedure consisted of placing 100 μL of each one of the samples diluted in oxalic acid at 0.4 %, plus 100 μL of acetate buffer (3 g of sodium acetate, 7 mL of distilled water and 10 mL of glacial acetic acid) and 800 μL of DCPI (24 mg/L) in eppendorf vials. After that, the samples were agitated in vortex, and absorbance was read at 520 nm. The standard curve was performed from 0 to 100 mg of ascorbic acid/L of oxalic acid at 0.4 % The results were expressed in milligrams equivalent to ascorbic acid per gram of paste in dry weight.
Determination of total carotenoid concentration
Total carotenoid concentration was quantified with the colorimetric method as described by Talcott & Howard, (1999) with slight modifications (Reyes et al., 2007). The procedure consisted of homogenizing 0.5 g of the sample with 12.5mL of acetone:ethanol (1:1) that contained 200 mg/L of BHT filtered through Whatman #4 paper and gauged at 50 mL with acetone:ethanol (1:1). To this extract, 25 mL of hexane were added, agitated and left to settle for 15 min. Subsequently, 12.5 mL of ultrapure water were added and left to settle for 30 min to allow phase separation. Absorbance of the hexane phase was read at 470 nm. Total carotenoid concentration was obtained from a standard curve from 0 to 30 µg β-carotene/mL. The results were expressed as micrograms equivalent to β-carotene per gram of paste in dry weight.
Determination of tocopherol concentration
Tocopherol concentration was performed following the methodology written by Pott et al. (2003) with slight modifications (Yahia et al., 2006); the sample (6 g) was homogenized with calcium carbonate (0.2 g) and methanol (15 mL), filtered and mixed with 50 mL of a hexane/acetone (1:1) mixture that contained BHT at 0.1 %. Then, it was agitated adding 40 mL of sodium sulfate at 10 % for phase separation. The upper layer was separated, washed several times with water and evaporated in a rotavapor at 35 °C. For saponification, the sample was dissolved in 30 mL of diethyl ether, 0.2 mL of methanol at 40 % of KOH, and the mixture was left for reaction in darkness at room temperature for 16 hours. After the saponification stage, the extract was washed with water and evaporated in a rotavapor at 35°C. To quantify α-tocopherol concentration, the colorimetric method was used as described by Kivçak & Akay, (2005). The results were expressed as micrograms equivalent to α-tocopherol per gram of paste in dry weight.
Determination of antioxidant activity of mango paste sub-product
Antiradical activity based on free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH•)
The antiradical activity based on DPPH• was evaluated according to the procedure reported by Morales & Jiménez-Pérez (2001). The antioxidant activity was expressed in μmol equivalent to Trolox/g paste p.s. (μmol ET/g paste p.s.). The technique consisted of preparing a DPPH• solution at a concentration of 7.4 mg/100 mL in ethanol and agitated for 10 min. Subsequently, 500 µL of the DPPH• solution were added to 100 µL of the samples in vials and agitated vigorously; they were left to settle at room temperature for one hour. After this time elapsed, the vials were centrifuged at 10,000 rpm at room temperature for five min; then, absorbance of the supernatant was measured at a wavelength of 520 nm.
A standard Trolox curve from 0 to 500 μmol Trolox/L was performed for each set of samples.
Antiradical activity based on capture of 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)
Capture capacity of the stable ABTS•+ cation was obtained following the ABTS (7mM) reaction in potassium persulfate (2.45 mM), incubated at 4 °C in darkness for 16 h. Once the radical ABTS•+ was formed, it was diluted with ethanol until an absorbance value of 0.70 (± 0.1) at 754 nm was obtained; 20 µL of the sample were added to 980 µL of the radical ABTS•+ dilution. Absorbance was measured at 754 nm after seven minutes of waiting period. Capture capacity of the free radical ABTS•+ was determined with a standard curve of ascorbic acid from 0 to 30 mg/100 mL; the results were expressed as mg equivalent to vitamin C (EVC)/g paste p.s. (Re et al., 1999; Kuskoski et al., 2005).
Reduction capacity of ion Fe (III) to ion Fe (II) (FRAP)
The reduction capacity of ion Fe (III to ion Fe (II) was determined by the method of Hinneburg et al. (2006), which consisted of taking an aliquot of 1 mL of each extract to mix it with 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium hexacyanoferrate [K3Fe (CN)6] at 1 %. After 30-min incubation at 50 ºC, 2.5 mL of trichloroacetic acid at 10 % were added and left to settle at room temperature for 10 min. Immediately, 2.5 mL of water and 0.5 mL of ferric chloride at 0.1% were added to the 2.5 mL assay to perform absorbance measurements at a wavelength of 700 nm. The ascorbic acid standard curve was made at concentrations from 0 to 15 mg/100 mL, and the results were expressed as milligrams equivalent to ascorbic acid (EAA)/g of paste in dry weight pasta.
Chelating activity
Chelating activity was analyzed with the method reported by Guicin et al. (2003); it consisted of placing 100 µL of the sample in 1.5-mL vials, adding 50 µL of ferrous chloride tetrahydrate solution (2 mM) and 450 µL methanol; assays were agitated and left to settle at room temperature for five min. Subsequently, 400 µL of ferrozine (5 mM) were added and left at room temperature to settle for 10 min. The calibration curve was performed with EDTA from 0 to 0.001 M/L; the results were expressed as micromoles equivalent to per gram of paste in dry weight.
Antioxidant activity based on the protection of β-carotene and linoleic acid emulsion
The protection activity of β-carotene and linoleic acid against oxidation was determined with the methodology reported by Vankar et al. (2006). It consisted of preparing an emulsion with 2 mL of β-carotene in chloroform (at a concentration of 0.6 mg/ mL) in a boiling flask; chloroform evaporated at 40 °C for 15 sec utilizing a rotavapor; then, 40 µL of linoleic acid, 400 µL of tween-20 and 100 mL of deionized water were added and agitated vigorously for one minute to form the emulsion. For the antioxidant assay, 200 µL of the samples were placed in test tubes; 5 mL of emulsion were added to each test tubes with the sample; they were agitated in vortex and submerged in water at 50°C. A BHT solution (4 mM) was used as protection standard against oxidation and deionized water as control. Readings were taken at 90 min of reaction, and absorbance was measured at 470 nm after tempering. A BHT curve of 0.0004 was performed at 4 mM/L. The results were reported in micromoles equivalent to BHT per gram of paste in dry weight.
Statistical analysis
The data of each parameter were tested for normality and homocedasticity. The results obtained were analyzed utilizing an analysis of variance (ANOVA) and Tukey’s test for multiple comparison of means when significant differences occurred among treatments with a significant level of 1 %. Likewise, the correlation between the bioactive compounds and antioxidant activity was established with Pearson’s test, utilizing SPSS (Statistical Package for Social Sciences) version 18.
Results
Concentration of bioactive compounds of mango paste sub-product
Figure 1A shows the total phenolic compounds (TPC) for the three cultivars of mango paste analyzed, observing that Ataulfo showed the greatest concentration with 37 mg EAG/g of paste p.s. (p<0.01).
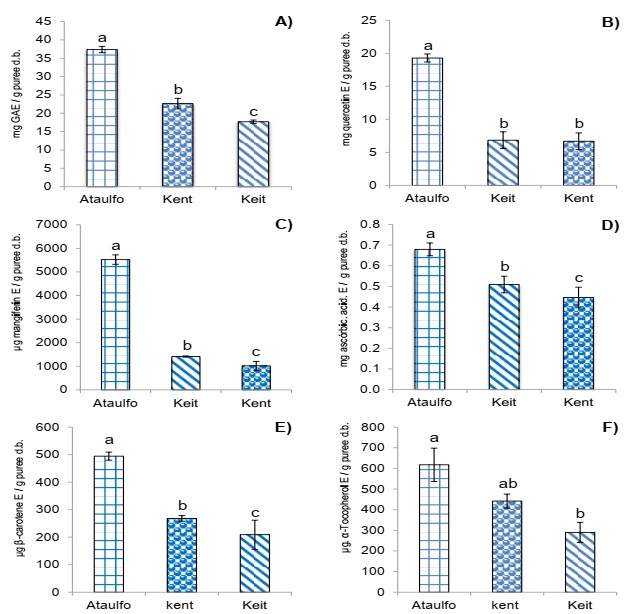
A) Concentration of total phenolic compounds, B) Concentration of total flavonoids, C) Concentration of mangiferin, D)Concentration of ascorbic acid, E) Concentration of total carotenoids, F) Concentration of total tocopherols. Results are expressed as means (columns) ± standard deviations (error barr) (n=3). Different letter (a, b, c) denote significant differences between treatments (p<0.01).
Figure 1 Concentration of bioactive compound in by-product puree of three varieties of mango.
Figure 1B shows the different flavonoid concentrations where the paste of Ataulfo obtained the greatest concentration with 19.3 mg E quercetin/g of paste p.s. while those of Kent and Keit cultivars obtained similar value between them with 6.7 and 6.8 mg E quercetin/g of paste p.s., respectively (p<0.01).
Figure 1C shows that the paste of Ataulfo had the greatest concentration of mangiferin with 5,523 µg E mangiferin/g of paste p.s., followed by Keit with 1,412 µg E mangiferin/g of paste p.s. and in last place, Kent with 1,018 µg E mangiferin/g of paste p.s. (p<0.01).
Figure 1D shows that Ataulfo has an average cultivation of 0.68 mg E ascorbic acid/gpaste p.s., which was significantly the highest of those observed for Keit and Kent cultivars (p<0.01).
Figure 1E shows carotenoid concentration where the three cultivars had different concentrations, of which Ataulfo was the highest with 494 µg E β-carotene/g of paste p.s and Keit the lowest with 209 µg E β-carotene/g of paste p.s (p<0.01).
Figure 1F shows total tocopherol concentration where Ataulfo showed the highest concentration with 620 µg E α-tocopherol/g of paste p.s. (p<0.01); this result of Ataulfo was greater than that reported by Corral-Aguayo et al. (2008), who determined that tocopherol concentration was 550 µg/100 g of pulp.
Antioxidant activity of mango paste sub-product
The capacity to capture free radical DPPH• of the different mango cultivars is shown in Figure 2A, observing that Ataulfo had the greatest antiradical activity with 244 µmol ET/g of paste p.s. while no differences (p<0.01) were found between Kent and Keit cultivars.
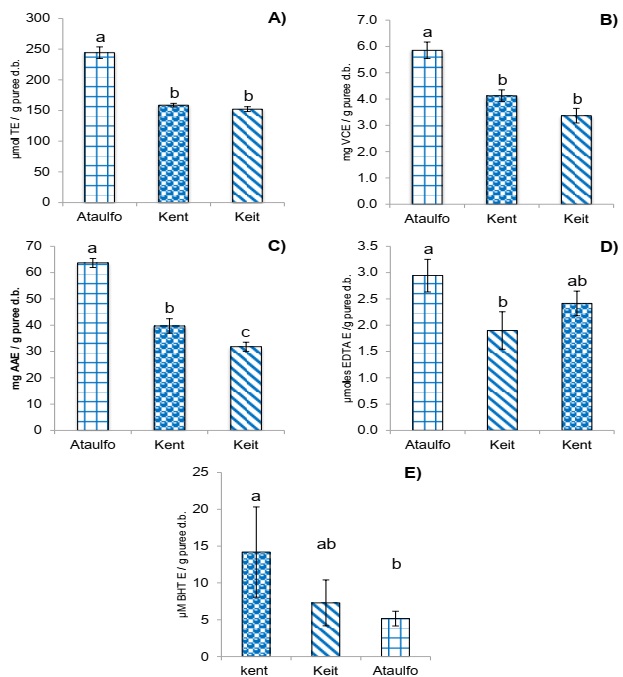
A) DPPH• radical scavenging activity, B) ABTS•+ radical scavenging activity, C) Ferric-reducing antioxidant power (FRAP), D) chelator activity, E) Antioxidant activity by β-carotene-linoleic acid method. Results are expressed as means (columns) ± standard deviations (error barrs) (n=3). Different letter (a, b, c) denote significant differences between treatments (p<0.01).
Figure 2 Antioxidant activity of by-product puree of three varieties of mango.
Figure 2B shows the antiradical activity results of mango cultivars on the ABTS•+ cation. Mango Ataulfo had the greatest antiradical activity of 5.9 mg EVC/g of paste p.s., followed by Kent and Keit cultivars without significant (p<0.01) differences between them.
The capacity of the three mango cultivars of reducing ion Fe (III) to ion Fe (II) is shown in Figure 2C, observing they had a different reduction capacity for Fe (III), of which Ataulfo showed the greatest activity with 64 mg E ascorbic acid/g of paste p.s., followed by Kent and last place for Keit with 40 and 32 mg E ascorbic acid/ g paste p.s., respectively (p<0.01).
The chelating activity of a complex extract is the result of the sum of the compounds that show this activity, such as ascorbic acid, carotenoids, anthocyanins and phenolic compounds (Martínez-Valverde et al., 2000; Ajila et al., 2007b).
Figure 1D shows the chelating activity of the analyzed cultivars, observing that Ataulfo had the greatest activity with 2.9 µmoles EDTA/g of paste p.s., and Keit had the least activity with 1.9 µmoles EDTA/g of paste p.s. while Kent was similar to both (p<0.01). Figure 2E shows that Kent cultivar had the greatest protection against emulsion oxidation with 14.2 µM E BHT/g of paste p.s. while Ataulfo had 5.1 µM E BHT/g of paste p.s., which was the least antioxidant activity; Keit cultivar was statistically similar to both cases (p<0.01).
According to the correlation analysis (Table 1) of the mango paste extracts analyzed, the capacity to capture radical DPPH• showed a positive correlation with the bioactive compounds analyzed, which was strong for total phenolic compounds, flavonoids, mangiferin, ascorbic acid and carotenoids while that with tocopherols was weak and less significant (p<0.05).
Table 1 Correlation of Pearson between antioxidant activity and bioactive compound of by-products puree of mango.
| DPPH• | ABTS•+ | FRAP | Chelator activity | β-carotene-linoleic acid method | |
|---|---|---|---|---|---|
| TPC | 0.93** | 0.42** | 0.93** | 0.17* | 0.06 |
| Flavonoid | 0.92** | 0.30** | 0.89** | 0.20* | 0.20* |
| Mangiferin | 0.94** | 0.32** | 0.87** | 0.25** | 0.21* |
| Ascorbic acid | 0.70** | 0.26** | 0.57** | 0.30** | 0.13 |
| Carotenoids | 0.89** | 0.40** | 0.90** | 0.12 | 0.07 |
| Tocopherols | 0.21* | 0.02 | 0.25** | 0.09 | 0.09 |
Results are expressed as R2 (n=3).
**Correlation significant at p<0.01 (bilateral).
*Correlation significant at p<0.05 (bilateral).
The antiradical activity against ABTS•+ had a weak (p<0.01) positive correlation with the majority of the bioactive compounds analyzed, except for tocopherols where no correlation was found.
For FRAP no strong positive correlations were found with phenolic, flavonoid, mangiferin and carotenoid compounds while the correlation was moderate with ascorbic acid and weak with tocopherols. This behavior was also reported by Corral-Aguayo et al. (2008) who found similar correlations.
Chelating activity was positively and weakly correlated with phenolic, flavonoid, mangiferin, and ascorbic acid compounds, of which these two last one were more significant than the first one. On the other hand, carotenoids showed a negative and weak correlation while no correlation was found with tocopherols.
In the case of oxidation protection with β-carotene and linoleic acid emulsion, the correlation analysis made evident that it was weak and negative with flavonoids and mangiferin while no correlation was found with the rest of the bioactive compounds. According to Kuskoski et al. (2005), the correlation of antioxidant activity with any compound not only depends on the concentration and quality of the same but also on its interaction with other product components. The low correlation of ascorbic acid and carotenoids with the different antioxidant activities analyzed could be related with the chemical characteristics of the molecule that determines their antioxidant properties, depending on the medium the reaction develops (Vrolijk et al., 2015).
Discusion
Concentration of bioactive compounds of mango paste sub-products
Manthey & Perkins-Veazie (2009), determined the concentration of total phenolic compounds in fruit of different mango cultivars (Tommy Atkins, Haden, Kent, Keit and Ataulfo) of different countries (Peru, Brazil and Mexico), reporting that mango Ataulfo from Mexico had the greatest CFT concentration with 109.3 mg EAG/100 g of purée. For the variety of mango Uba (Brazilian), 208.7 mg EAG/100 g dry weight have been reported (Ribeiro et al., 2007). The results obtained in this determination for the three different mango paste analyzed were greater than those reported by Berardini et al. (2005) and Abdalla et al. (2007) who found 406.6 mg EAG/100g in peel and 112 mg EAG/100 g seed, respectively. The main flavonoids reported in mango are mangiferin, catechin, epicatechin, quercetin, isoquercetin (quercetin-3-glucoside), antocyanins, fisetin and astragalin (kaempferol-3-glucoside) (Masibo & He, 2008).
The values obtained in this research for the concentration of ascorbic acid were found within the interval reported in different research studies, where a great variation was observed from 0.09 to 1.86 mg/g mango pulp (Franke et al., 2004; Gil et al., 2006; Ribeiro et al., 2007; Reyes et al., 2007; Corral-Aguayo et al., 2008; Manthey & Perkins-Veazie, 2009). On the other hand, the content of ascorbic acid in skin has been reported by Ajila et al. (2007b) with an average of 0.34 mg/g p.s.; these results are lower than those obtained in this research study.
Same as with other fruit, mangoes differ in their ascorbic acid content due to genotype, climate factors and maturity stage. Lee & Kader (2000) reported that at the beginning of the maturity stage, the content of ascorbic acid was greater and it decreased with time (Lee & Kader, 2000). Sample preparation and the analytical methods used for quantification also have an influence (Ribeiro & Schieber, 2010).
Chena et al. (2004) determined 25 carotenoids in mango pulp; trans- β-carotene was present in the greatest amount (29.34 µg/g), followed by its isomer cis- β-carotene (9.86 µg/g), violaxantin and its isomer cis (6.40 µg/g), neochrome (5.03 µg/g), luteoxantin (3.6 µg/g), neoxantin and its isomer cis (1.88 µg/g), zeaxantin (1.16 µg/g) and 9-or 9′-cis-luteina (0.78 µg/g) (Masibo & He, 2009).
Mango pulp contains a wide spectrum of carotenoids, including β-carotene, violaxantin, criptoxanthin, neoxantin, luteoxantin, and zeaxantin (Ornelas-Paz et al., 2007); β-carotene is mainly the predominant carotenoid in mango constituting 48-84 % of the total carotenoids (Manthey & Perkins-Veazie, 2009), which has been reported in a range from 1,159 to 3,000 mg/100g of pulp from different mango cultivars (Pott et al., 2003; Chena et al., 2004; Ornelas-Paz et al., 2007).
Ornelas-Paz et al. (2007) reported carotene values of seven different mango cultivars harvested in Mexico from 0.4 to 7.7 mg/100g of sample, of which Haden had the greatest concentration of carotenoids (7.7 mg/100 g of sample) while Ataulfo and Kent showed an average of 2.4 and 3.2 mg/100g of sample, which agrees with the results obtained in this study.
The values obtained in this research study in total tocopherol concentration of the three analyzed mango paste were within the range of the values reported by Charoensiri et al. (2009) for total tocopherol concentration of the main mango cultivars consumed in Thailand (Kheosawoei, Nahmdawgmai and Rad with values of 0.35 and 1.45 mg/100 g mango pulp, respectively). Ornelas-Paz et al. (2007) reported values from 200 to 500 µg/100g pulp of seven mango cultivars harvested in Mexico (Haden, Ataulfo, Tommy Atkins, Manila, Kent and Paraiso, Criollo variety), of which Tommy Atkins and Haden obtained the highest contents with 500 and 399 µg/100g pulp, respectively.
In other cultivars, such as Glechoma longituba, a high variation was found in total flavonoid concentration and other bioactive compounds, depending on geographic origin; likewise, a positive correlation was found between soil iron and phosphorous, temperature and humidity as key factors in bioactive compound variation (Jin et al., 2018). This same effect was detected in gallic (Allium sativum L.) cultivation where genotype, several cultivation practices, as well as post-harvest conditions were determined to influence on gallic bioactive potential (Martins et al., 2016). Likewise, a study described that in Passiflora setacea cultivation, phenolic compounds and vitamin C concentrations were greater in rainy seasons that in dry ones, particularly in the case of vitamin C, finding that even cultivation techniques could influence its concentration (De Carvalho et al., 2018).
Antioxidant activity of mango paste sub-product.
Manthey & Perkins-Veazie (2009) determined the capacity of five mango cultivars (Tommy Atkins, Haden, Kent, Keit and Ataulfo) of different countries (Peru, Brazil, Ecuador and Mexico) to capture radical DPPH•, reporting that mango Ataulfo of Mexico showed the greatest activity with an average of 104.2 mg E gallic acid/100 g purée, attributing this activity to the concentration of total phenolic compounds of this cultivar (109.3 mg E gallic acid/100 g purée). According to the results of Khammuang & Sarnthima, (2011) and Soong et al. (2004) a positive correlation exists between total phenolic compounds and antioxidant activity of mango seed.
Moreover, mangiferin content is also related to the capture capacity of the radical DPPH•, existing evidence that it is a strong antioxidant and chelator of metal ions (Shah et al., 2010).
With respect to the capacity of capturing stable ABTS•+ cation, Kuskoski et al. (2005) determined the antioxidant activity as equivalent to vitamin C of tropical fruit pulp and reported 224.7 mg EVC/100g in mango pulp sample. Maisuthisakul & Gordon, (2009) analyzed the antioxidant activity of Chok-Anan mango seed by the ABTS•+ method, showing an activity of 0.75 mmol of Trolox/g sample. Dorta et al. (2012) reported an antiradical activity of 16.7 g TE/100 g p.s. in skin and 11.5 g TE/100 g p.s. in seed of mango Keit.
The phenolic and carotenoid compounds and vitamin C in mango skin and seed are good donors of electrons that can reduce Fe3+ to the ferrous form (Chung et al., 2002), indicating such capacity as that of the analyzed mango paste.
The results obtained in the chelating activity of the analyzed mango extracts agree with that suggested by Pitchaon (2011), mentioning that metal chelating activity is the highest in accordance to phenolic content. Maisuthisakul & Gordon (2009) reported that mango embryo of Chok-Anan cultivar showed a chelating activity slightly greater (68.4 µg/mL) compared to gallic acid (65.5 µg/mL) in in vitro assays with ferrous ion (Fe2+) complex and ferrozine, which also indicated that such activity was related to the concentration and structure of the phenolic compounds present in the extract.
Maisuthisakul & Gordon (2009) assessed the effect of drying (sun and oven) different solvents (water and ethanol) on the antioxidant activity of Chok-Anan mango seeds. They found that mango seed extracts protected against linoleic acid oxidation in 71.0 ± 6.4 at 105 ± 3.9 AA50 (antioxidant activity) which were more efficient than cumarin with 94.9 ± 0.1.
In response to an oxidative imbalance, cells produce antioxidants of different chemical nature, as defense molecules, can eliminate or neutralize free radicals. When mango fruit is exposed to a low UV light flux (0.6 J cm-2) the phytochemical content may increase 130 % in pulp and 197 % in skin. Likewise, total antioxidant activity in pulp increases up to 130 % and 145 % in skin. However, vitamin C is only preserved 40 % in pulp while the content decreases up to 50 % in skin (Lopes et al., 2016).
On the other hand, hot water treatments (as pest control) in a temperature range from 46-47 °C and time from 5-90 min have been identified to regulate the transcriptions that codifies the supposed adhesion proteins of cell surface, transcription factors sensitive to ethylene, Arabic proteins, stress responses, cytochrome p450, kinases for the processes related with defense, fatty acid metabolism, flavonoids and biosynthesis of volatile esters, as well as genes associated to photosynthesis (Dautt-Castro et al., 2018).
Conclusions
The paste obtained from mango pulping sub-products of Ataulfo cultivar showed both the greatest antioxidant activity and highest bioactive compound concentration, of which total phenolic compounds were the main constituents. Based on statistical correlation results, we may conclude that antioxidant activity of Ataulfo paste was the main function of total phenolic compound concentration.











 texto en
texto en 



