Introduction
Putrescine (Pu), Spermidine (Spd) and Spermine (Spm) polyamines (PAs) are ubiquitous molecules essential for the growth and survival of organisms (Kusano et al., 2008); as well for the replication, transcription, translation, modulation of ionic and receptor binding channels, and for the stabilization of many glycoproteins (Brooks, 2013). PAs are conserved from bacteria to animals and plants with some variations in their biosynthetic pathways (Tabor & Tabor, 1984). The synthesis essentially starts from L-arginine and L-methionine precursors (Smirnova et al., 2018). Although genes encoding for the enzymes, which participate in PAs metabolic pathways and metabolome, are known (Panicot et al., 2002), the dynamics of its expression during environmental changes has not been investigated yet. However, the importance of PAs participation in stress response is obvious, due to the increasing number of research works showing changes in PAs levels associated to diverse kinds of stress (Smirnova et al., 2018; Satish et al., 2018; Majumdar et al., 2017; Valdés-Santiago & Ruiz-Herrera, 2014). Total intracellular concentration of PAs is in the range of various hundreds of micromoles to a few millimoles and is tightly regulated, since the highest levels of PAs are toxic for cells and drive to cellular death (Dong-Hun et al., 2018). PAs levels are regulated in various steps including de novo synthesis, degradation and transport; as well, limiting enzymes of its metabolism are used to sustain distinct effects due to stimuli they are subjected to (Ou et al., 2016), making them adequate regarding other less economical molecular markers used in P. capitata (Delgadillo-Nuño et al., 2014; Liñán-Cabello et al., 2010a; Liñán-Cabello et al., 2010b). In vegetal tissues the accumulation of Reactive Oxygen Species (ROS) and PAs during abiotic and biotic tensions are considered as healers of stress-induced damage and active elements of signaling cascade of such phenomenon, more than being collateral consequences of metabolic changes (Shabala & Pottosin, 2014). Therefore, its levels could be associated to the physiological state of organisms, depending on environmental conditions in which they are.
Since depth, and therefore irradiation, affect productivity in coral reefs (Lesser et al., 2000), studies with molecular markers associated to the survival of these organisms in environmental changes are required (Buckley & Szmant, 2004). Among other physicochemical and optical characteristics, light incidence has been observed to be altered, including in Photosynthetically Active Radiation (PAR, 400-700 nm) in sites where the tide falls below the normal average (Banaszak & Lesser, 2009), like in Playa Mora, one of the sampled sites in this study. Moreover, the submarine light field is modified by the incident light angle, light absorption and dispersion by dissolved materials, as well as by particles in water. As well, the illuminated environment is an important component of coral reef productivity, physiology and ecology (Lesser et al., 2000). In symbiotic zooxanthellae found in coral reefs, there are microsporine-like amino acids (MAA), working by absorbing UV radiation between 330 and 360 nm, in which a probable similar function to the one of PAs has been described, and causing the loss of a methyl group, when analyzing them with an ion trap and in standard conditions (Whitehead & Hedges, 2003). The present study tries to determine Pu, Spd and Spm levels in three areas of the Central Mexican Pacific (CMP) coast with coral reef development of P. capitata, subjected to extreme variations in sea level. The objective is to assess the concentration of such PAs in P. capitata and the possible differences in the levels of these compounds of coral reefs, obtained under environmental conditions in two contrasting periods of the year, February, in which sea level significantly falls, and October, in which corals are always immersed in water. In the particular case of Playa Mora, the coral development of this site remains emerged during February, therefore environmental effects may be more obvious, compared to both systems of Pocillopora genus in Chamela Bay, located in always immerged sites.
Materials and Methods
To assess health status of P. capitata coral populations in CMP coasts, using PAs as molecular markers, the branch coral was sampled in two extreme environmental conditions: winter, when tide level presents the maximum lowest tide of the year and at the end of summer and beginning of autumn, when tide levels are at their highest during the year or maximum full tide. Moreover, diverse attributes were considered in the selection of coral colonies for sampling. Among them, in order of importance, there were: the greenbrownish color of the colony, visual indicator of health, free of epibionts, and some apparent damage visually detected. Based on the afore-mentioned, eight fragments were sampled, between 5 and 10 cm in length for the branch of randomly selected colonies at a depth of 1.8 to 3.5 m in Piedra Bola (19°34´31.6” N, 105°07´59.4” W), Palmito (19°33´0.4” N, 105°06´20.7” W) and Playa Mora (19°16´49” N, 104°52´20” W) in February and October of 2014, with the help of SCUBA diving, latex gloves, hammer, chisel and dark plastic bag with Ziploc-type zipper. Samples were stored at 4 °C until its transfer to the Laboratory of Biotechnology of the University Center of Biological and Agricultural Sciences and stored at -20 °C for its further analysis.
The identification of P. capitata coral was realized under anatomical and structural criteria of the exoskeleton of each fragment with the help of taxonomical keys of Hodgson (1995) and Reyes-Bonilla et al (2005).
Chamela Bay
It has an extension of 2,364 km2; geologically here is the Cocos plate, subduction ridge and narrow platform, with cliffs, islands and jetties. In Chamela Bay, two sites were selected (Figure 1), to realize sampling of P. capitata coral fragments: Piedra Bola, with two big coral reefs separated from each other by a sandy area; and Palmito with isolated coral growth of Poritas sp.
Tenacatita Bay
It has an extension of 6.5 km and three main beaches are identified: Manzanilla, Tenacatita and Boca de Iguanas. The selected site is Playa Mora which is located at the northwestern tip of the bay.
Tide level in CMP. Tide register
Monthly values of tide level for CMP were obtained from databases of the Station of Manzanillo port, Colima, Mexico. A period of five years was considered, from 2012 to 2016. The statistical analysis of the values of the tides was performed for the months of February and October of the five years.
Laboratory Analysis
Sample extraction
Eight replicas were cut from the 1 cm2 fragments of coral for each sampling site and were macerated with 10 mL of acetone at a concentration of 90 % with distilled water as a solvent, they were left to rest for 2 h and the obtained supernatant was centrifuged at 6,000 rpm for 15 min. Later, 30 μL of diaminoheptane (1 nmol/µL) was added as an intern standard, and they were left to airdry and at room temperature for 7 days. Later, they were resuspended in 300 μL of acetonitrile (AcN) and then centrifuged (Labgene 1730R) at 14,000 rpm for 20 min at 4 °C. Forty μL of bicarbonate 20 mM, pH 12, 100 μL of sample supernatant and 100 μL of dansyl chloride (SigmaAldrich) were added in amber flasks, it was let overnight in darkness, then dried again with N2 gas at 60 °C (dry bathLabnet) and resuspended with 100 μL of AcN to take it to Solid-Phase separation.
Equipment and chromatographic conditions
The HPLC system (VARIAN, Melbourne, Victoria, Australia) was used. It was equipped with SupelcosilTM LC18 column (30 x 0.4 cm x 5 μm, Supelco, Bellefonte, PA, USA); UV-Vis detector (Varian 9050). The mobile phase used was an AcN-water solution (90 %-10 %) with a 1 mL/ min flow. Spectrophotometric detection was performed at 450 nm and total time of the analysis was of 13 min.
Solid-Phase separation
To activate the cartridge (Hypersil ODSC18), it was passed through 1 ml of methanol, then 1 mL of bicarbonate 20 mM, pH 12. Later, 1mL the resuspended sample in AcN was added and it was washed with 5 mL of HPLC-grade water. Sample was eluted 1.0 mL de can/min, at 26 °C. Finally, 20 μL of sample were injected by duplicate in the chromatograph, all solvents were previously filtered. PAs recovering test was performed, recovering more than 80 % of Pu, Spd and Spm (data not shown).
Statistical analysis
Results were presented as x ± sd and were analyzed by means of a normality test at 95 % of confidence. Data were assessed by means of an analysis of variance (ANOVA) of two factors, using experimental variables, site and tide levels (mean for February and October for 5 years) pursuing with a Duncan’s Multiple Range Test, to determine differences by site and tide level. Statistical analyses were realized with STATGRAPHICS Centurion XVII statistical software (©2014 Statpoint Technologies, Inc.).
Results and Discussion
Nowadays there are no reports determining PAs levels in coral. For this reason, we report some relevant aspects of the chromatographic study, such as the retention times for the three analyzed PAs, which were: 3.64 min for Pu; 5.88 min for Spd and 9.51 min for Spm. For the lower detection limit, 35 pg/mL was determined for Pu; 62 pg/mL for Spd and 45 pg/mL of Spm. For the upper limit detection, it was assessed up to a concentration of 80 µg/mL.
Although such molecules are considered as stress bioindicators in plants and one of the main molecular markers in this kingdom (Majumdar et al., 2017); PAs participation and its changes in response to stress (Smirnova et al., 2018; Satish et al., 2018; Majumdar et al., 2017; Valdés-Santiago & Ruiz-Herrera, 2014), make them a potential alternative to other indicators used in coral. However, more studies are required to allow to establish them as true stress-related indicators in such organisms.
A general evaluation of experimental data, show that the presence of PAs, Pu, Spd and Spm, in P. capitata coral tissue significantly varies among sites (Palmito, Piedra Bola and Playa Mora) and sampling time (February and October). Similarly, PAs concentration was significantly higher in October. If the presence of the different PAs is compared (Figures 1-3). The overall average was: 10.03 µg/cm2 of Pu, 1.24 μg/cm2 of Spd and 4.06 of Spm, with lower limits of 1.35±0.16 µg/cm2 in Pu, 0.15±0.03 μg/cm2 in Spd and 0.07 ± 0.02 µg/cm2 in Spm and upper limits of 42.91 ± 1.40 µg/cm2 in Pu, 5.31 ± 1.06 µg/cm2 in Spd and 12.10 ± 0.75 µg/cm2 in Spm. It is important to establish, the relation in PAs contents in P. capitata coral regarding studies reporting PAs levels in distinct tissues and/or fluids of animals, since there are no reports regarding corals. For instance, Khuhawar & Qureshi (2001) used concentrations at levels of µg/mg of creatine in urine; and the review by Teti et al. (2002) showed values raging from nmol/mL of simple, not only for urine, but also for plasma, pmol/µL in red blood cells, urine and cells of lymphoma, as well as μg/mL in serum and ng/μL in concentrated urine. In such a way that these levels of concentration in corals, equivalent to that of animals and plants, would allow estimates to be reached, including at the cellular level, both coral and symbiont or zooxanthellae.
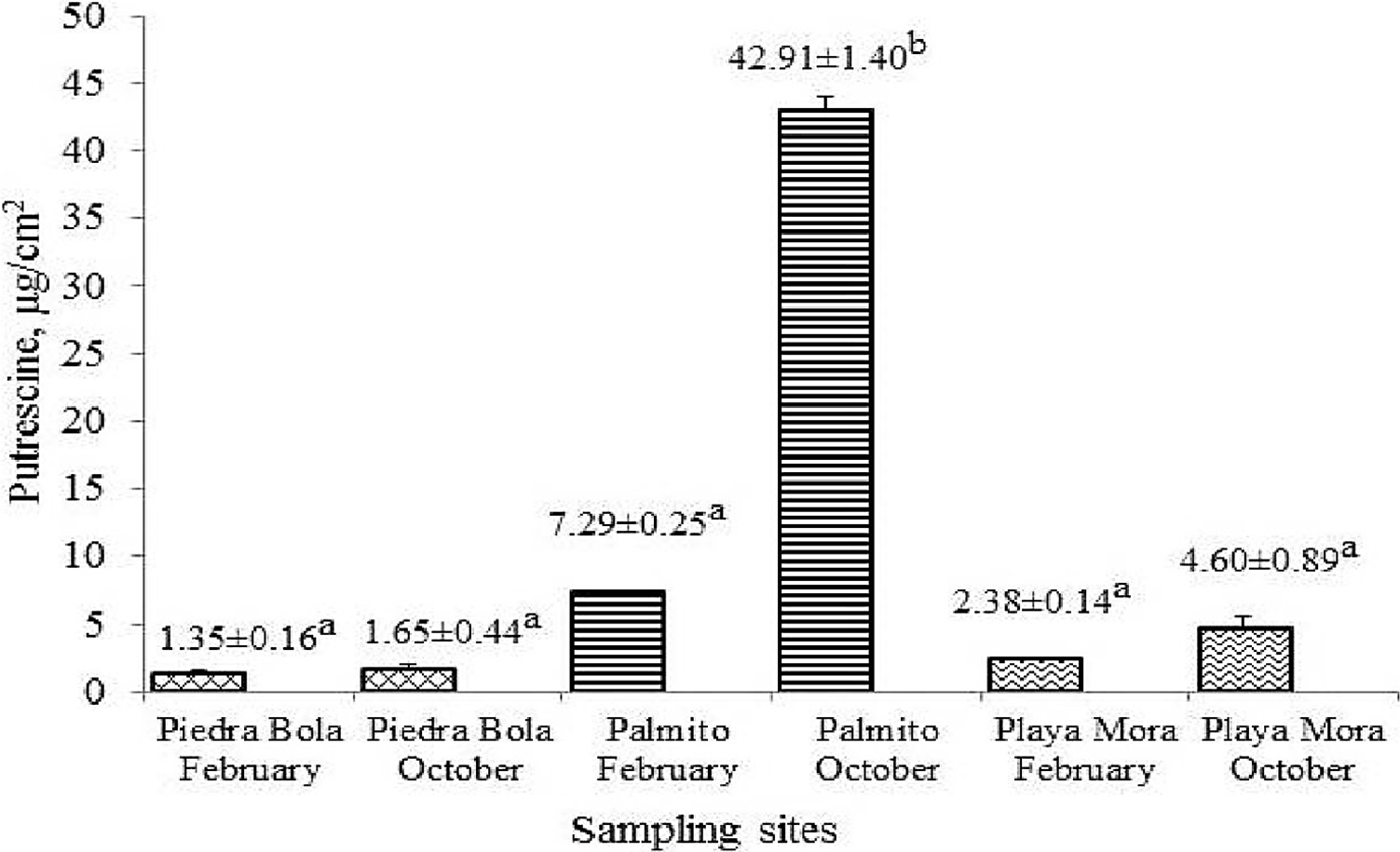
Figure 2 Concentration of putrescine (μg/cm2) in P. capitata tissue during February and October in the three sampling sites. Different letter indicates significant differences (p<0.05).
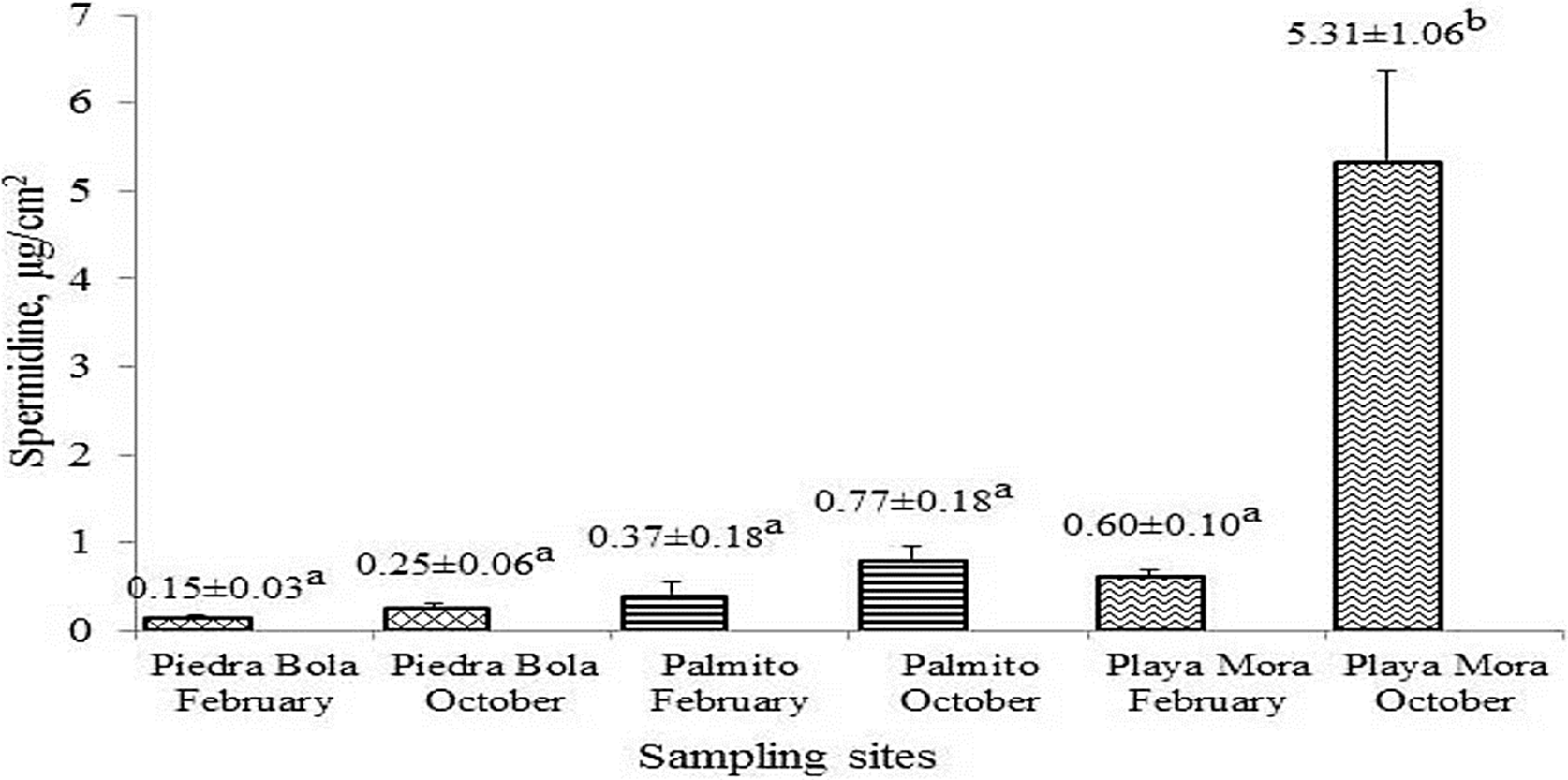
Figure 3 Spermidine concentration (μg/cm2) in P. capitata tissue during February and October in the three sampling sites. Different letter indicates significant differences (p<0.05).
Regarding sampling sites, in Palmito, Pu was observed to present the highest concentration, in various orders of magnitude, among PAs recorded by site and month. In contrast, Playa Mora and Piedra Bola, in October, presented the highest concentration of Spm, while Playa Mora showed the highest concentration of Spd. When comparing tide level by sampling month, which is one of the oceanographic factors that draws attention the most, the height of the water column is significantly lower in February (-0.31 ± 0.02 m regarding the annual average tide height) than in October (0.05 ± 0.01 m regarding the annual average tide height).
A discussion on the particular individual cases is made.
Pu concentration is observed in Figure 2. This polyamine presented significant differences among sampling sites, but not among sampling periods or tide level (Psite = 0.0102; Ptide level = 0.375), recording the highest concentrations in Palmito, with 7.29±0.25 µg/cm2 in February and 42.91±1.40 µg/cm2 in October.
In relation to Spd, the highest concentrations were presented in October (Figure 3). Significant differences were established among sampling sites, but not among sampling months or tide level (Psite = 0.0167; Ptide level = 0.307). Playa Mora showed the highest concentrations with 0.60±0.10 µg/cm2 in February and 5.31±1.06 µg/cm2 in October.
Spm (Figure 4) registered significant differences among sampling months or tide level (Ptide level=0.0439). The highest concentrations were in October, with 12.10±0.75 µg/cm2 in Playa Mora, and 5.33±0.88 µg/cm2 in Piedra Bola.
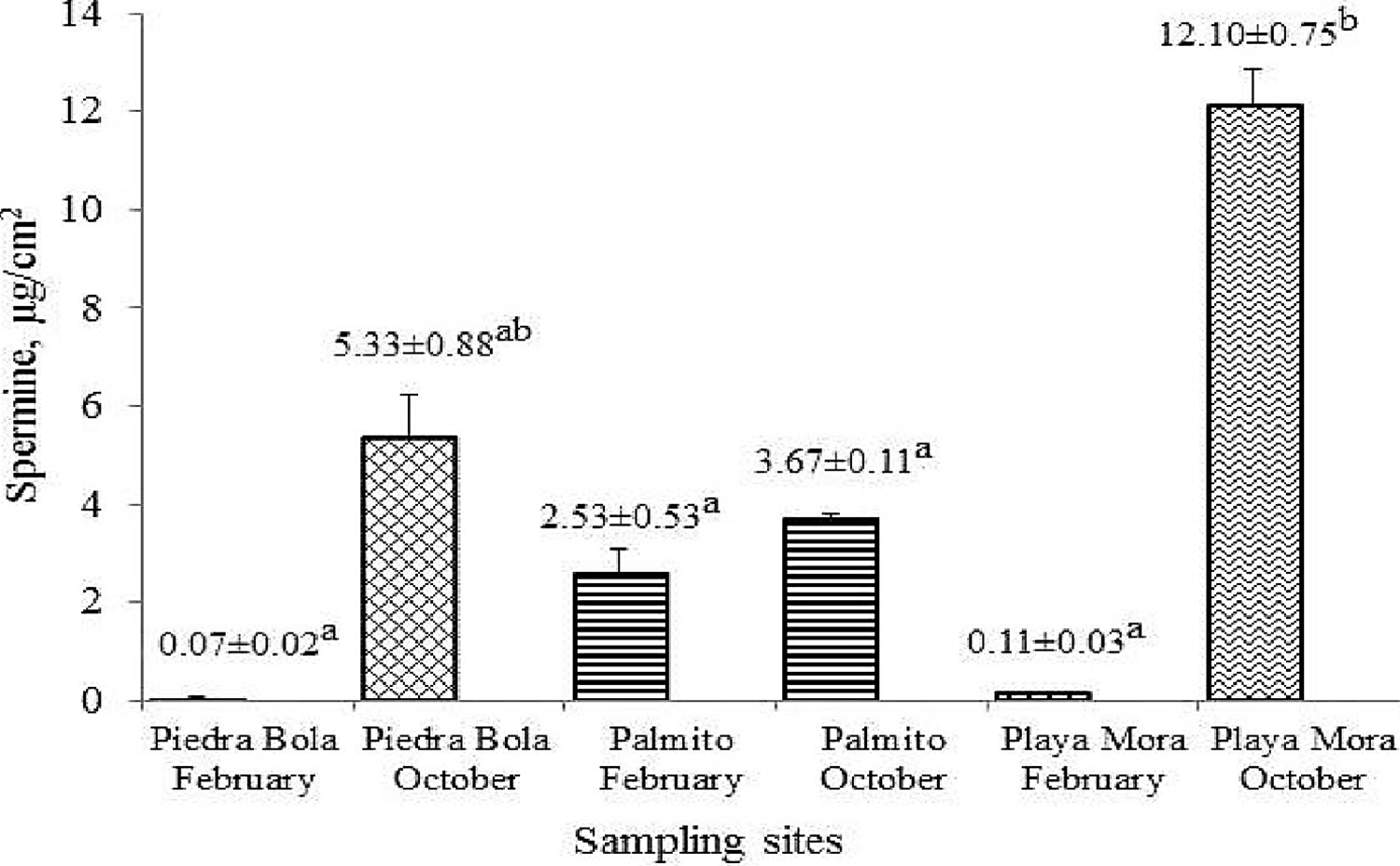
Figure 4 Average Spermine concentrations (μg/cm2) in coral tissue P. capitata recorded at sites and months of sampling. Different letter indicates significant differences (p<0.05).
Changes in tides were recorded from the tide station of Manzanillo port, for 5 years in the period comprised between 2012 and 2016 and averaging the 12 months of each year (Figure 5). By means of a non-parametric Kruskal-Wallis test and Dunn’s multiple comparison test (p≤0.001), results showed very marked significant differences among the first 4 months of the year, compared to September, October and November months, especially in February and October months when sampling of the present research work was performed and in the three analyzed sites.
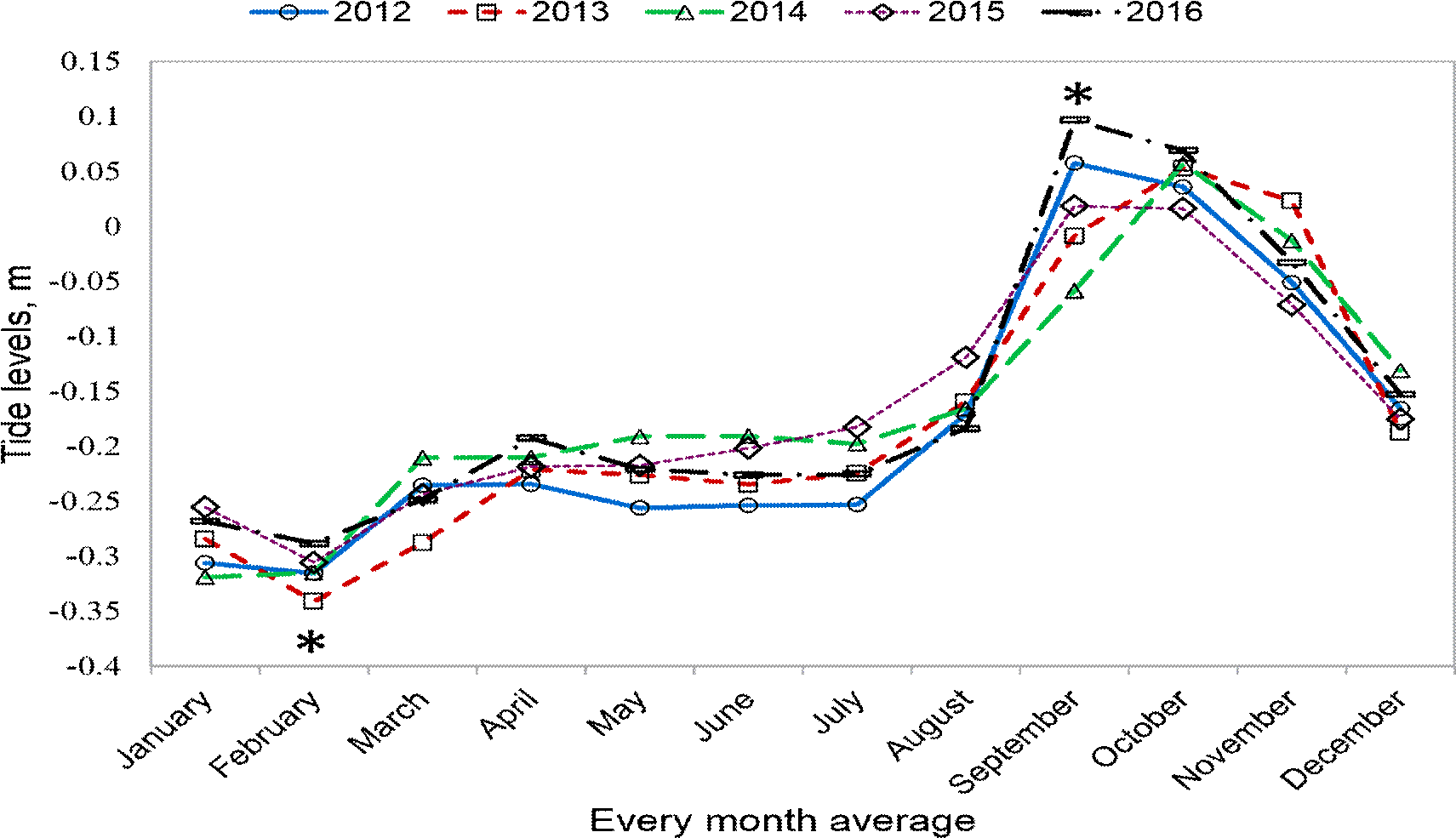
Figure 5 Tide levels in monthly average, during 5 years. The asterisks show that there is a statistically significant difference by the ANOVA test and multiple comparison, p<0.001.
Results obtained in the present study suggest PAs, specially Spd and Spm, as markers associating PAs levels with tides, which could indicate a proper homeostasis for all metabolic pathways to be fully operational, as well as replication, transcription and protein synthesis, while Pu over-accumulation induced apoptosis (Tobias et al., 1995; Teti et al., 2002), although more studies are required with other stress control indicators, like chlorophylls, carotenoids, nutrients and/ or biomass present in P. capitata (Buckley & Szmant, 2004). Biological systems are generally complex and have mechanisms operating at different levels, molecular, cellular or population (Downs et al., 2005). Consequently, an adequate quantity of indicators is required (Buckley & Szmant, 2004) to determine the stress degree in which they are, implying that they are representative of the structure, function and composition of the ecological system and that the information they bring can be complemented in a whole analysis (Adams, 2005). It is important to take into account that some chemical and cellular markers appeared in short periods of time or after an impact, and others indicate a cumulative effect for lengthy periods of time (Hughes et al., 2003).
Given the multiple functions attributed to PAs, it is difficult to establish the origin of Spd and Spm, accumulated in this coral species from Paya Mora, since it could be the characteristic metabolism of such molecules, its exacerbation and/or probably some relation with Micosporinas (MAAs) metabolism for defense, given not only in artificial conditions (Whitehead & Hedges, 2003) but also in natural ones as well, where they may interfere with the donor of methyl groups through the S-adenosil metionina (SAM) (Brooks, 2013). It is known as well that PAs have direct effects on ion transport at membrane scale in mammals (Li et al., 2007; Kusano et al., 2008). Due to the null research work conducted on the metabolism of these molecules in corals, physiological studies on these molecules are required to be able to propose them as stress indicators (Buckley & Szmant, 2004) as it is accepted for plants (Liu et al., 2017).
On the other hand and importantly, associating PAs levels with some other parameters of the ecosystem results difficult since many factors may influence in the space where corals are. If primary productivity is taken as a reference for stress in the zone where the present study was performed, it is found that in low tides period, which is in February, the best productivity of coral biomass is registered, synonym of lower stress and PAs levels without important changes or its association with the physiological status of the organisms in symbiosis (Teti et al., 2002).
Another alternative could be the association of tide levels, since tide level has an effect on other factors impacting the coral system, such as the incidence of solar radiation, turbulence associated to the water column and turbidity or clarity of it, to name a few; and stress indicators, although those already established for this type of study, act as a response to more demanding environmental conditions (Sosa-Avalos et al., 2006). In such a way that Spd and Spm can represent an adaptive mechanism to conditions of less productivity around October, if considered as a stress condition, increasing its concentration to favor metabolic pathways of stress indicator molecules, like MAAs and given the vast relation of PAs precursor amino acid, specifically ornithine (Sivashanmugam et al., 2017), in this model, but studies in the same model and PAs levels are required, in addition to those already established indicators.
The present study performed in natural conditions on coral located in CMP region, and since they are exposed to diverse light regimes according to depth and location in the reef (Liñán-Cabello et al., 2010) there are not many previous studies. P. capitata, one of the two most abundant coral species in the zone of study (Reyes-Bonilla et al., 2013), shows an adequate physiological adaptive mechanism to seasonal changes (Liñán-Cabello et al., 2010). Moreover, it presents, at an enzymatic level, a series of responses focused on adaptation and maintenance of its reproductive capacity in oceanic shallow environments, which usually exhibit high levels of ultra-violet radiation, in addition to tide influence, short-term changes in turbidity, nutrient concentration, temperature and osmolarity that can act in combination and cause irreversible damages (Liñan-Cabello et al., 2010). All or some of these conditions can be modified when tides reach a difference of approximately 35 cm, like in the case of coral zones studied in February and October (Figure 5). Spm concentration was significantly different among sampling periods; the sampling site had a higher effect on Pu and Spm concentrations, 42.91 and 12.1 µg/cm2 respectively. The obtained results showed a significant higher PAs concentration in October than in February, as well as differences among sites, the highest Spd and Spm levels being registered in Playa Mora, while the highest Pu value was expressed in Palmito, probably by activation of PAs retro-conversion pathway (Dong-Hun et al., 2018). These differences are not explained only by tide levels, since the studied systems are submitted to diverse environmental factors. On one hand, in October, when the tide is at its highest, corals can be prone to the turbulence and pressure of a higher water column, to solar radiation with a lower inclination and to higher environmental and seawater temperatures, as well as to a higher water transparence, favoring a lower dispersion of solar rays. On the other hand, conditions in February and part of March, with lower tide level, to such a degree that even in Playa Mora, coral mass remains exposed to air, and consequently, does not receive pressure and turbulence of the water column, and the environmental conditions showed a lower temperature of these months of the end of the winter, as well as a higher turbidity, which could disperse solar rays (Sosa-Avalos et al., 2006).
Conclusions
There is a relation between PAs levels in P. capitata and tide levels considered as stress inductors in CMP beaches, assessed in the present study. The present work reports, for the first time, the concentration of diverse polyamines in P. capitata coral tissue, obtained in coral development from the CMP. The possible use of these molecules is suggested as tools to determine the health status of organisms from the most diverse and complex ecosystem of the planet, as coral reefs are.











 texto en
texto en 




