Introduction
Selenium (Se) is an essential element for animals and humans. Se organic compounds increase antioxidant defense, reducing susceptibility to diseases such as cancer and other disorders related to oxidative stress (Yasin et al., 2015; Schiavon et al., 2016; Mimmo et al., 2017). Furthermore, Se acts as a co-factor of enzymes involved in several biochemical pathways of plants and animals (Ahmad et al., 2016); thus, plant-based food is an important source of Se for humans. On the other hand, Se is not considered an essential element for plants, but it is a beneficial element, inducing tolerance at low doses to different environmental factors that generate oxidative stress, including tolerance to arsenic in rice (Singh et al., 2018; Chauhan et al., 2017), to cadmium and lead in canola (Wu et al., 2016), to saline stress in tomato (Mozafariyan et al., 2016) and to water deficit in corn (Nawaz et al., 2016). Selenium decreases the adverse effects of oxidative stress, inducing different components of the enzymatic and non-enzymatic antioxidant systems of plants, as well as the activity of GSH-peroxidases, increasing the ascorbic acid, glutathione, non-protein thiol and phenolic compound levels (Singh et al., 2018; Chauhan et al., 2017; Srivastava et al., 2009). In addition, Se is used as fertilizer to increase its content in the edible part of crops, such as carrots, lettuce (Smolen et al., 2016), corn (Nawaz et al., 2016) and radish (Schiavon et al., 2016). Thus, the application of this agronomic strategy, known as biofortification, contributes to crop production and to greater health benefits. Additionally, an increase in corn yield and grain quality (Nawaz et al., 2016) and a higher content of nutraceutical compounds (glucoraphanin) in radish roots (Schiavon et al., 2016) and polyphenolic compounds in strawberries (Mimmo et al., 2017) with Se supplementation have been reported.
In the soil, Se exists in four oxidation states: selenite (SeO3 2-, Se4+), selenate (SeO4 2-, Se6+), elemental Se (Se0) and selenide (Se2-) (Krystyna, 2002). Plants can absorb Se in the form of SeO3 2- or SeO4 2-; they are the most common inorganic compounds present in the soil solution, water and most foods (do Nascimento da Silva et al., 2017).
Unlike Se, vanadium (V) has been less studied in plants, although it is also considered a beneficial element (Trejo-Téllez et al., 2016). However, V has been identified as a micronutrient for other living organisms (Nalewajko et al., 1995). This element is part of several metalloenzymes, such as nitrogenases (in Azotobacter chroococcum) and haloperoxidases. In humans, V complexes perform a wide variety of biological activities, such as insulinomimetics and antitumor activities, and act as enzyme inhibitors (Habala et al., 2015).
V is generally not very mobile in the soil, and it has several cationic states, including V2+, V3+, V4+, and V5+; the latter form is characterized by being a strong oxidant (Matsugo et al., 2015). V4+ is the predominant form in the soil, which is less mobile and toxic than the pentavalent (V5+) form (Tian et al., 2015). Small amounts of V stimulate plant biomass production, whereas root and leaf growth inhibition and morphological changes have been reported with high levels of this element in Ipomoea aquatica (Chen et al., 2016). Conversely, the dry weight of basil (Ocimum basilicum) roots increased as the V dose increased (Akoumianaki-Ioannidou et al., 2016). In cucumber, the V could have an antagonistic effect on the uptake of toxic heavy metals, such as lead and cadmium, as well as a synergistic effect on the uptake of essential nutrients to plants (Charles & Onyema, 2016).
Green peppers, which include jalapeño, bell pepper, poblano, serrano, and chilaca, represent one of the vegetables with the highest economic value in Mexico (SIAP, 2017). In addition to its commercial value as a fruit, pepper is important for its content of bioactive molecules such as capsaicinoids, provitamin A, vitamin C and folate, which have a positive influence on human health (Guzmán & Bosland, 2017). In other crops, such as radish (Raphanus sativus L.), the roots are the main organ that is consumed, although leaves, seeds, and flowers can also be consumed; this species is rich in potassium, zinc, glucosinolates, and antioxidant compounds such as flavonoids, anthocyanins, and vitamins C and B (Schiavon et al., 2016).
Based on the potential uses of the beneficial elements in agricultural production, the aim of this work was to evaluate the effect of V and the two oxidation states of Se (Na2SeO3 and Na2SeO4) more commonly taken up by plants on the germination and the initial growth of serrano pepper and radish seedlings.
Material and methods
Homogenous commercial seeds of serrano pepper (Capsicum annuum L. cv. Chiltepec) and radish (Raphanus sativus L. cv. Champion) without apparent damage were selected.
Establishment of the experiment
Transparent polypropylene containers with lids (500 mL) were used, and a layer of filter paper moistened with 8 mL of each treatment was placed at the bottom of the container. Subsequently, twenty seeds of pepper or radish were placed in each container. The treatments consisted of 1.25, 2.5 and 5 μM Se as Na2SeO3 or Na2SeO4, 2.5, 5 and 10 μM V (NH4 VO3), and distilled water (0 μM) as a control. The Se and V sources used in the experiments were analytical grade (Sigma-Aldrich; Saint Louis, MO, USA). The containers with the seeds were placed in a growth chamber at 25 ± 2°C with a 16 h light/8 h dark photoperiod under fluorescent white light (32 μmol s-1 m-2). Two independent experiments were established with three replicates for each treatment (n=6), and each replicate consisted of a container with 20 seeds. Experimental units were arranged in a completely randomized design with 10 treatments.
Evaluated parameters
Germination
In the case of serrano pepper, germination was monitored every three days for up to 17 days after the establishment of the experiment, whereas the number of germinated seeds in radish was recorded daily until six days after the application of treatments. In both cases, a seed was considered germinated when the radicle reached 2 mm in length. This parameter was expressed as a percentage.
Seedling growth
The seedling height and root length were determined with a graduated ruler. The number of roots was recorded when differences between treatments were observed. The dry weight of the seedlings was obtained after drying the plant tissue at 72 °C for 48 h (four grouped seedlings were considered as one replicate per treatment). This parameter was determined 15 and 24 days after the application of treatments in radish and serrano pepper seedlings, respectively.
Results and discussion
Germination
Selenium, in the form of selenite (Na2SeO3) or selenate (Na2SeO4), increased the germination percentage of serrano pepper (Figure 1). The maximum germination percentage was obtained in the treatment with 2.5 μM of selenite or selenate (Figure 1D). These results differ from those reported by Frias et al. (2009), in which selenite decreased the germination rate of Lupinus angustifolius, but results from the same doses (2, 4, 6 and 8 mg L-1) of selenate were statistically similar to that in the control (0 mg L-1). In contrast, no differences were found between the Se ionic forms evaluated in this study at a low dose (less than 1 mg L-1). Germination rates at 2.5 μM (≈ 0.4 mg L-1) of selenite or selenate were higher than those observed in the control. On the other hand, the germination rates in the different treatments with V were similar to those in the control (Figures 1C and 1D).
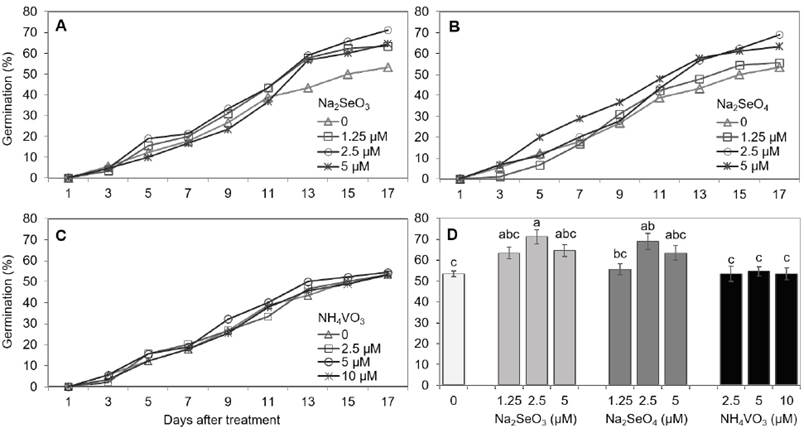
Figure 1 Germination percentage of serrano pepper seeds under different concentrations of Na2SeO3 (A, D), Na2SeO4 (B, D) and NH4VO3 (C, D). The data shown in subfigure D represent the comparison between treatments on the last data recording day (day 17). Means with different letters indicate statistically significant differences according to Duncan’s test (p < 0.05), ± standard deviation.
The radish seeds showed fast germination, within 24 h of experiment establishment, without differences among all treatments evaluated with Se or V (Figure 2). Müller & Engelbrecht (2017) also found no significant effects on the germination of Medicago polymorpha and Trifolium subterraneum seeds with the application of several selenate concentrations (from 0.25 to 4.0 mg L-1, ≈ from 1.3 to 21 μM). Similarly, vanadate application (0.1 μM) had no influence on germination, growth, or metabolism (carbohydrates, proteins, photosynthetic pigments, photosynthesis, and respiration) in rye and wheat (Kasai et al., 1999). It is noteworthy to highlight that the dose used in the latter study was low compared with the concentrations used in this work (1.25, 2.5 and 5.0 μM).
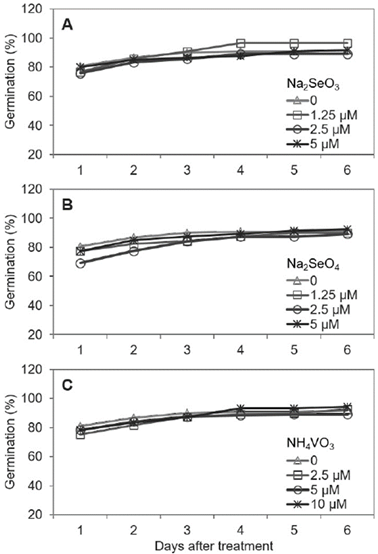
Figure 2 Germination percentage of radish seeds under different concentrations of Na2SeO3 (A), Na2SeO4 (B) and NH4VO3 (C). Statistical differences between treatments are not significant (p > 0.05).
Conversely, Ahmed (2010) reported a decrease in the germination of lettuce, tomato and radish seeds as the Se concentration increased from 10 to 200 mg L-1 Na2SeO4. These results indicate that several aspects must be considered when using beneficial elements such as Se and V in agriculture. For example, high concentrations could inhibit germination and growth because these elements are not essential for plants, but low doses, such as those used in this study, can increase the percentage of germinated seeds, as in serrano pepper (Figures 1A, 1B, 1C and 1D), or have no effect on germination, as in radish (Figures 1C and 2).
Another important aspect to highlights from the results found in this study was the species-dependent effect of beneficial elements. Thus, determining the correct ionic form and the suitable concentration range of the beneficial elements for each species is necessary to enhance the desired characteristics in the crop.
Seedling growth
The shoot growth of serrano pepper seedlings was differentially stimulated depending on the Se ionic form, obtaining the highest seedling height in the selenite treatment, with similar results at the three doses evaluated in this study. The seedlings grown with selenate or vanadate showed a height similar to the control (Figure 3A). All Se and V doses increased the root length to almost double the growth observed in the control plants (Figure 3B). This effect on root growth has also been reported in Brassica oleracea, since low concentrations of V (1 mg L-1 as VOSO4) favored radicle elongation, whereas concentrations higher than 3 mg L-1 caused severe symptoms of toxicity and root growth inhibition (Kaplan et al., 1990). Additionally, Se or V application induced root formation, obtaining higher root number compared with those in the control, although no differences were observed among the treatments with V and the two sources of Se (Figure 3C). Consistent with these results, Vachirapatama et al. (2011) observed lateral root emergence in B. campestris ssp. chinensis (Chinese green mustard) plants grown at 20 and 40 mg L-1 V. This root growth stimulation in serrano pepper seedlings would be advantageous for their establishment in the field.
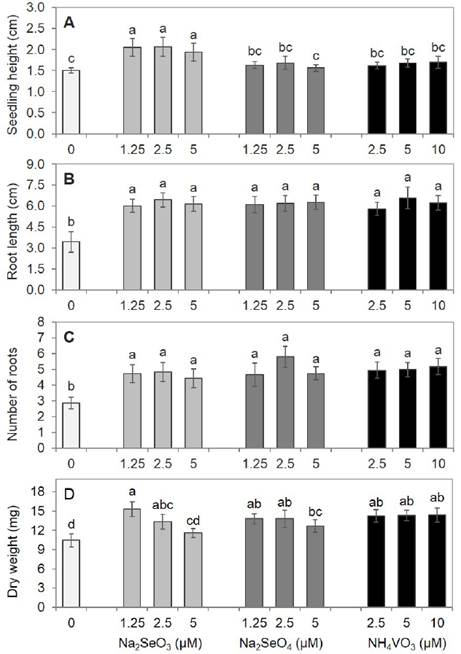
Figure 3 Seedling height (A), root length (B), number of roots (C) and dry weight (D) of serrano pepper seedlings after 24 days of experiment establishment. Means with different letters indicate statistically significant differences according to Duncan’s test (p < 0.05), ± standard deviation.
The pepper seedlings treated with 1.25 μM selenite showed a 47 % increase in dry biomass, but this increase percentage decreased as the dose increased to 5 μM, which resulted in dry weight values similar to those in the control and the 5 μM selenate treatment (Figure 3D). Similarly, low Se dose (2 and 6 mg L-1) in Na2SeO3 promoted growth and increased the photosynthetic pigment content in Oryza sativa L. ssp. japonica, as well as the expression of proteins related to primary metabolism and photosynthesis, indicating that the beneficial effect of Se on growth could be attributed to the positive regulation of carbohydrate and protein metabolism in the root and the increase in photosynthetic efficiency in the aboveground parts (Wang et al., 2012). The dry weight values of seedlings treated with V were statistically similar to those in the selenate treatments and the two lowest concentrations of selenite, but higher than those in the control, mainly due to roots development. Our results contrast with those reported for B. campestris ssp. chinensis, where growth changes were not observed in plants exposed to low V doses (1 and 10 mg L-1), whereas doses higher than 20 mg L-1 (≈ 170 μM) drastically decreased the leaf, stem, and root dry weight (Vachirapatama et al. 2011). In this study, an increase of 38% in the dry weight of pepper seedlings was found at similar V concentrations (10 μM ≈ 1.2 mg L-1). Akoumianaki-ioannidou et al. (2015) reported that V improved the uptake of some essential elements such as Fe that are involved in chlorophyll biosynthesis and as a co-factor of enzymes that participate in the photosynthetic electron transport chain and contribute to growth stimulation.
Although the germination percentage of radish seeds was not modified by any treatment (Figure 2), the application of beneficial elements had positive effects on seedling growth. The seedling height values were similar in the treatments with selenite, selenate and vanadate, and in all cases were higher than those of the control (Figure 4A). The tallest seedlings were those treated with 2.5 μM V, although the seedling height values were statistically similar to those from the other treatments with V, selenate, and the highest selenite concentrations. The growth of the primary root was only stimulated by the addition of 5 μM of selenite in comparison to root growth in the control (Figure 4B). No differences were observed in the root number among treatments (data not shown). For seedling dry weight, only the lowest concentrations of selenate and V favored its accumulation, without any difference in seedling dry weight among the other treatments and the control (Figure 4C). The same trend was observed with Se and V in other species; both elements reduced the dry weight as their concentration increased in the nutritive solution. Vachirapatama et al. (2011) reported a negative effect on dry biomass accumulation in B. campestris ssp. Chinensis with high V concentrations (40 and 80 mg L-1), where dry biomass was lower than that observed in the control. The results of this study showed that concentrations lower than 1 mg L-1 (1.25 μM ≈ 0.30 mg L-1) stimulated seedling growth (dry weight) in radish, with a decrease in the dry weight as the V concentration increased until the values were statistically similar to those in the control (Figure 4).
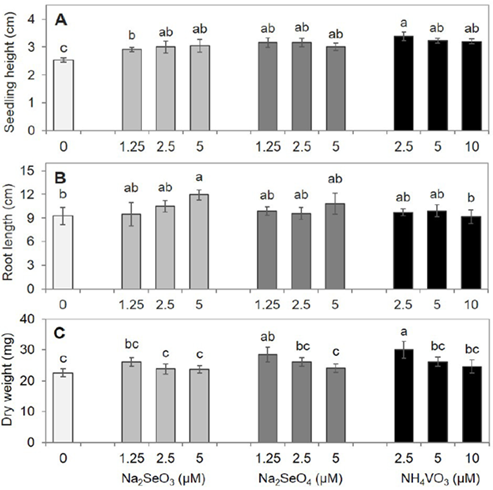
Figure 4 Seedling height (A), root length (B) and dry weight (C) of radish seedlings after 15 days of experiment establishment. Means with different letters indicate statistically significant differences according to Duncan’s test ( p < 0.05), ± standard deviation.
In this study, differential effects of Se and V were observed on germination and the seedling growth of serrano pepper and radish. Se application improved the germination rate of serrano pepper seeds, but it had no effect on radish germination. In contrast, seed germination was not influenced by V in either species (Figures 1 and 2). Se and V also had a greater effect on serrano pepper growth, which indicates that the beneficial effect of these elements is a function of the species. Consistent with this finding, Arscott & Goldman (2012) reported a differential effect of Se on growth in broccoli, mung beans and onion sprouts with different harvest dates (harvest 1: day 3 for broccoli and mung beans, day 5 for onion; and harvest 2: days 5 and 7, respectively). The seeds were treated with several selenate doses (0, 127, 635, 1270 and 12700 μM), and significant differences were not found among the three species evaluated on the first harvest date. In the second harvest, the biomass production in broccoli was reduced by 45 % at doses from 635 to 12700 μM. In mung beans, there were no statistically significant differences among treatments, and a negative effect was found on onion at the highest selenate concentration. Therefore, the anatomical differences between dicotyledons (broccoli and mung beans) and monocotyledons (onion) could influence the Se uptake capacity and metabolism. Similarly, the addition of Se (such as Na2SeO4) increased the seedling height and dry biomass accumulation of M. polymorpha, whereas the opposite effect was observed in T. subterraneum seedlings at the same Se concentrations (Müller & Engelbrecht, 2017). This response to Se and V in a species-dependent manner could be due to the different abilities of species to take up and accumulate these chemical elements in their tissues, resulting in changes in plant growth and development.
Conclusion
Se and V differentially affected the germination process and the growth of serrano pepper and radish seedlings. Se increased the germination percentage of serrano pepper seeds, whereas V had no effect on the germination of either species. Although both elements promoted the seedling growth of species evaluated in this study, it is confirmed that low doses of Se and V have positive effects on germination, growth or both processes of the plant life cycle. However, in the case of Se, no differences were found between the application of selenite (Na2SeO3) or selenate (Na2SeO4) for germination, but there were different effects on the initial growth of serrano pepper and radish seedlings. Therefore, the beneficial effects of these chemical elements can be applied in agriculture but require taking into account that their use depends on the concentration, source, crop type, application method, exposure time, and growth stage.
Further studies are needed to generate knowledge about the effect of these chemical elements at other growth stages and on fruit quality, as well as their role in counteracting plant stress and their effects on human health as a result of crop biofortification.











 texto en
texto en 



