Introduccion
The Crassulaceae family is the most representative of Mexico due to the high endemism of most of its species and to its historical-cultural significance and for the role they play in the structure and function of arid ecosystems of the country (Toledo, 1988); they present a high genetic diversity, which appears with variation in plant height, size, shape, thickness and color of the leaves, size and shape of the inflorescence (Borys et al., 2005).
Mexico is considered as the second center of diversity with a representation of approximately 300 (89.9 %) endemic species (t’ Hart, 1997), being this percentage of endemism the highest among families and genera in this country (Thiede, 1995). Mexico is an important place of high diversity in vegetal species (Colinas, 2003).
Due to the incomparable shape and beauty of its flowers, Crassulaceae are prized and valued on the international market; the aforementioned causes an intense and illegal harvest and sacking of plants, fruits and seeds, with the purpose of selling and recording them in other countries (Bravo-Hollis, 1978). Conservation and use of genetic resources is of strategic importance for humanity; the different vegetal species have been submitted to an active interaction with the environment, which caused a high number of genotypes adapted to different local conditions, expanding genetic diversity (Becerra & Paredes, 2000).
In the state of Morelos, 70.5 % of the families of vascular plants of Mexico are present, 36.4 % of the genera and 14.2 % of the species. These numbers reveal that the state is an important reservoir of diversity for all vegetal groups and at distinct levels of taxonomical hierarchy (Rendón & Fernández, 2007).
Morphological characteristics have been being used to study genetic diversity, identify cultivated plants and conserve genetic resources (Yasmin et al., 2006), as well as the method of molecular characterization measuring genetic variability directly at the scale of the DNA molecule (Watson & Eyzaguirre, 2002). Molecular markers have been become important tools in studies on genetic diversity, as RAPDs (Bered et al., 2005 and Yasmin et al., 2006).
Associations of genetic markers by means of cluster analysis are considered to be very useful since they include a high number of characters and because it is a hierarchical classification that can be used to determine threshold levels of similarity among species (Singh, 1999). As well, they indicate the formation of very distinct clusters to those considered from the classical approach based on the morphological and anatomical analysis (García, 2003).
The description and characterization of Crassulaceae by Eggli (2003) and Acevedo-Rosas et al. (2004) were based on morphological traits. Van Ham et al. (1994) used universal primers to amplify DNA by PCR of three non-coding regions of chloroplastic DNA with the purpose of studying the variation in length of the DNA sequence in Crassulaceae and related species. Similarly, Van Ham & t’ Hart (1998), analyzed a restriction zone of chloroplastic DNA of 44 species of six Crassulaceae subfamilies, using 12 restriction endonucleases in order to establish phylogenetic relations among them.
Morphological and molecular analysis in Crassulaceae is of high importance to know the genetic diversity and the relation among genotypes, since this will allow a correct classification, genotype conservation and genetic resources utilization. Currently, there is a high number of collections of germplasm with genotypes of high agronomical value which could be used as progenitors in breeding programs; however, in many occasions their degree of diversity and the relation among materials are unknown, making their utilization difficult. Genetic resources in Mexico have not been properly exploited due to limited available data on the characteristics and genetic diversity of many species. (Becerra & Paredes, 2000).
Based on the aforementioned, the objective of the research work was to evaluate genetic diversity and relations by performing the morphological and molecular characterization by RAPDs of twelve species of the Crassulaceae family. This characterization will allow to generate complementary information for plant breeders, and for a future utilization of genetic material to perform interspecific crosses.
Material and Methods
Studied genetic material were twelve species of the Crassulaceae family cultivated in pot: Echeveria runyonii topsy-turvy (Haworthia x E. Shaviana) (Mexico) (Maatz, 2012), E. perle von Nuremberg (E, gibbiflora var. metalica x E. potosina) (Mexico and San Luis Potosi), E. agavoides Lem. (San Luis Potosi), E. pumila var. glauca x E. Walther (Mexico), Echeveria elegans Rose (Mexico), E. pulvinata (Puebla), E. derembergii (Mexico) E. setosa Rose & J.A. Purpus (Puebla), Pachyphytum oviferum J.A. Purpus (San Luis Potosi), Sedum clavatum (Mexico), S. nussbaumerianum Bitter (Mexico), S. palmeri S. Watson (Mexico) (Sajeva & Constanzo, 1997) (Figure 1).

Figure 1 Studied species of the Crassulaceae family. a: Echeveria runyonii topsy-turvy, b: E. perle von nuremberg, c: E. agavoides Lem., d: E. pumila var. glauca x E. Walther., e: E. elegans Rose, f: E. pulvinata, g: E. derembergii, h: E. setosa Rose & J.A. Purpus, i: Pachyphytum oviferum J.A. Purpus, j: Sedum clavatum, k: S. nussbaumerianum Bitter, l: S. palmeri S. Watson.
Materials were obtained from commercial nurseries, they were selected by size, height, color and shape of the leaves, since these species stand out for their decorative value, as well as for the capacity they have to produce shoots, in addition of being some of the most commercialized species. Thirty plants were assessed for morphological analysis, of each indicated species.
Morphological characterization
Assessment of 30 plants was performed when flowering. Units of measurements in cm were measured with a thirty-centimeter ruler and a digital Vernier to obtain mm; and the color of organs was assessed with a color chart (RHS color chart®). Flowers were observed under microscopy to evaluate the number of stamens and carpels (Table 1). Some characteristics pointed out by Martínez-Ávalos & Mora-Olivo (2000), García-Ruíz & Pérez-Calix (2007) employed for the description of some species of the Crassulaceae family were used.
Table 1 Qualitative and quantitative morphological traits assessed in twelve species of the Crassulaceae family.
| Traits | Quantitative | Qualitative |
|---|---|---|
| Form of the plant | Height (cm) and Diameter (mm) | Rosette and Branch |
| Stem | Number of sheets | Phylotaxy |
| Leaves | Length (cm) and width (cm), thickness at the base and middle part (mm) | Apex shape, Petiole or pseudopeciolus, Color |
| Inflorescence | Number per plant, length (cm), flowers per inflorescence, peduncle length (cm), peduncle diameter (mm). | Origin: basal, middle, apical, ramifications: primary, secondary. Type of inflorescence. |
| Bracts | Number, length (mm), width (mm), base thickness and middle part (mm). | Phylotaxy, bract shape, apex shape and base shape |
| Flower | Length (mm), basal diameter (mm) and pedicel length (mm). | |
| Chalice | Form, Free or merged | |
| Separations | Number of sepals, length (mm) and width (mm). | |
| Corolla | Diameter (mm) | Shape |
| Petals | Number | Color |
| Stamens | Number | |
| Carpels | Number, and length of carpels (mm) | Free or merged |
| Time of flowering | Date |
Molecular characterization
DNA was obtained from one plant per species. Four hundred mg of foliar tissue of each one of the twelve species were weighted, to extract DNA according to the methodology reported by Andrade et al. (2004); at the last step, the obtained DNA was dissolved into 25 µL of 0.1 TE (1 mM Tris-HCl pH 8.0, 0.1 mM EDTA) con 20 ng∙µL-1 of RNAse, heating at 37 °C for 40 min. and stored at -20 °C until analysis.
DNA integrity in all plants was assessed by means of electrophoresis in 1 % agarose gel (ultrapure GIBCO®), gel was dyed with ethidium bromide (1 µg mL-1). In gel wells, a 9 µL volume was loaded, constituted by 1 µL of DNA sample, 7 µL of distilled deionized autoclaved water and 1 µL of loading buffer (40 % glycerol, 0.1 % bromophenol blue, TE pH 8.0). Electrophoresis was realized under room temperature with TAE buffer (0.04M Tris-base, glacial acetic acid, 1 mM EDTA) for two hours, time in which the reference band migrated 7.0 cm approximately. After this time, DNA samples were visualized and photo-documented in a gel analyzer (Syngene® GVM20).
DNA concentration and purity were quantified from the obtained DNA solution, for each sample a 1:150 dilution was prepared [5 µL of sample DNA more 995 µL of TE 10x (10 mM Tris HCl pH 8.0, 1 mM EDTA pH 8.0)], to quantify DNA concentration and purity by means of a spectrophotometer Genesys 6® of ultra-violet light. Concentration was assessed according to the formula:
Where:
OD260 = Optical density of DNA solution read at 260 nm wavelength.
DF = Dilution factor.
50 µg µL-1 = DNA concentration determined for a value of 1.0 a 260 nm.
Purity was assessed as the proportion of readings at wavelengths of 260 and 280 nm (260/280). Values between 1.8 and 2.0 of optical density (OD) indicate a high degree of purity. Values lower than 1.8 indicate contamination of the DNA sample with proteins and/or other elements absorbing ultraviolet light; while higher values indicate contamination by chloroform, phenol or other organic substance. Once readings for each DNA sample were obtained, working solution at 20 ng µL-1 were prepared.
RAPDs analysis
DNA Amplification (TECHNE TC-412®) was performed with 68 primers (Operon Technologies Inc.) of randomized sequence of ten nucleotides from the OPA, OPB, OPC kit, plus eight indicators: SAP, out of these, 20 primers were selected (Table 2), based on quantity and brightness of amplified RAPDs fragments, which were used to perform the study of genetic similarity.
Table 2 Sequence of the twenty primers selected for the molecular characterization of twelve species of the Crasulaceae family.
| Primers | Key | Sequence of bases |
|---|---|---|
| 2 | OPA02 | 5´ TGC CGA GCT G 3´ |
| 4 | OPA04 | 5´ AAT CGG GCT G 3´ |
| 7 | OPA07 | 5´ GAA ACG GGT G 3´ |
| 8 | OPA08 | 5´ GTG ACG TAG G 3´ |
| 9 | OPA09 | 5’ GGG TAA CGC C 3’ |
| 10 | OPA10 | 5´ GTG ATC GCA G 3´ |
| 1 | OPB-01 | 5´ GTT TCG CTC C 3´ |
| 6 | OPB-06 | 5´ TGC TCT GCC C 3´ |
| 7 | OPB-07 | 5´ GGT GAC GCA G 3´ |
| 8 | OPB-08 | 5´´GTC CAC ACG G 3´ |
| 10 | OPB-10 | 5´ CTG CTG GGA C 3´ |
| 12 | OPB-12 | 5´ CCT TGA CGC A 3´ |
| 18 | OPB-18 | 5´ CCA CAG CAG T 3´ |
| 19 | OPB-19 | 5´ ACC CCC GAA G 3´ |
| 8 | OPC08 | 5´ TGG ACC GGT G 3´ |
| 9 | OPC09 | 5´ CTC ACC GTC C 3´ |
| 13 | OPC13 | 5´ AAG CCT CGT C 3´ |
| 16 | OPC16 | 5´ CAC ACT CCA G 3´ |
| 25 | SAP-03 | 5´ TGG GAC CTC C 3´ |
| 26 | SAP-04 | 5´ GGA GCT ACC T 3´ |
Reaction mix consisted of 10 µL of dNTPs (5 µM of each dNTP, GIBCO BRL), 2.5 µL of PCR buffer (10x), 1.5 µL MgCl2 (3 mM), 2.0 mL of initiator (10 pmol µL-1), 0.3 µL (1.5 U) of native DNA taq polymerase (Invitrogen), 4 µL of DNA (20 ng µL-1), adjusting to 25 µL with 4.7 µL of deionized sterilized water.
The program used for amplification by means of polymerase chain reaction (PCR) consisted in an initial cycle of DNA pre-denaturalization at 94 °C for 4 minutes and 35 cycles integrated by the following steps: 94 °C for 1 min to separate DNA strands, 36 °C for 1 min for primer alignment, 72 °C for 2 min for extension or polymerization and a cycle of final extension at 72 °C for 10 min.
The separation of amplified fragments was made by means of electrophoresis in 1.5 % (p/v) agarose gel (Invitrogen) with ethidium bromide (10 µg L-1). The electrophoresis was performed at 75 V for 4 h. Gels with amplified DNA fragments were photo-documented in an Electrophoresis Documentation and Analysis System (Syngene GVM20®, Kodak®).
Statistical analysis
The size of DNA fragments produced by RAPDs primers was obtained using Gene tools Labworks 4.0® software. The presence of RAPDs markers was determined by means of the analysis of the electrophoretic profiles generated for each one of the Crassulaceae species. For dendrogram construction, data were analyzed, based on the presence (indicated by 1) or the absence (indicated by 0) of amplified fragments with the selected decamer primers.
Morphological and molecular data were processed using the Numerical Taxonomic and Multivariate Analysis System (NTSYSpc 2.1). Distance coefficient used for clustering was the Euclidian distance. Cluster analyses were realized on the relation of matrixes with Unweighted Pair Group Method with Arithmetic Mean method (UPGMA) (Avise, 1994). NTSYS software proposes cut-off point in the dendrogram according to a congruence assessment comparing with a tridimensional graph of projection to observe the number of formed groups.
Results and Discussion
Morphological characterization
Qualitative characters allowed to observe two types of characteristics in the studied species, the first one are the discriminative characteristics with which each one of the species are characterized and make them distinctive, expressing Crassulaceae morphological diversity; growth habit, shape and color of the leaf, origin and type of inflorescence, shape of bracts, and color are included in these characteristics; the second type of characteristics are those of similarity shared among these species, like apex and base shape, inflorescence ramification, phylotaxy of bracts, calyx shape, corolla and petals shape, carpels and flowering time, since they are qualitative characters, a multivariate analysis was not run.
The four first main components explained 81 % of the observed morphological variability. Principal component 1 (PC1) with an eigenvalue of 9.795 explained 40.81 % of the variation, where the variables that most contributed were leaf length, thickness of the base of the leaf, thickness in the medium part of the leaf, diameter of the peduncle, bract length, flower length, basal diameter, sepal length, corolla width and carpel length. Principal component 2 (PC2) explained 21.07 % and the variables that described this variation within this component are inflorescence length, peduncle length, bract width, thickness of the base of the bract, thickness of the medium part of the bract. In principal component 3 (PC3), height, number of leaves and number of flowers per ramification variables are those that explained 13.37 % of the total variability. In principal component 4 (PC4), length of the pedicel variable explained 6.13 % of the variance (Table 3).
Table 3 Proportion of global variance, vectors and eigenvalues of the principal components analysis (PC) of the 24 morphological traits describing variation in 12 Crassulacea species.
| Trait | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Height | 0.481 | 0.289 | 0.699 | 0.216 |
| Diameter | -0.659 | -0.463 | -0.304 | -0.286 |
| Number of sheets | -0.180 | -0.325 | -0.728 | 0.255 |
| Leaf length | -0.679 | -0.528 | 0.062 | -0.263 |
| Sheet width | -0.613 | -0.563 | 0.208 | -0.358 |
| Thickness at the base of the leaf | -0.848 | -0.404 | 0.141 | 0.022 |
| Thickness in the middle part of the leaf | -0.844 | -0.116 | 0.004 | 0.189 |
| Number of inflorescences | 0.068 | -0.372 | -0.337 | -0.167 |
| Inflorescence length | -0.506 | -0.819 | -0.054 | 0.112 |
| Number of flowers per branch | -0.336 | -0.411 | 0.768 | -0.179 |
| Pedicle length | -0.396 | -0.780 | 0.058 | 0.255 |
| Pedicle diameter | -0.771 | 0.351 | -0.131 | -0.200 |
| Number of bract | -0.379 | -0.566 | 0.634 | -0.172 |
| Bract length | -0.836 | 0.126 | 0.261 | 0.295 |
| Bract width | -0.655 | 0.594 | 0.326 | -0.221 |
| Thickness of the base of the bract | -0.523 | 0.666 | 0.341 | -0.084 |
| Thickness of the middle part of the bract | -0.593 | 0.662 | 0.227 | 0.034 |
| Flower length | -0.863 | 0.036 | -0.089 | 0.400 |
| Basal diameter | -0.834 | 0.220 | -0.383 | -0.017 |
| Pedicel length | -0.485 | -0.125 | 0.249 | 0.719 |
| Sepals length | -0.744 | 0.450 | -0.238 | -0.041 |
| Sepals width | -0.636 | 0.390 | -0.386 | -0.074 |
| Corolla width | -0.847 | 0.276 | -0.291 | -0.092 |
| Carpels length | -0.679 | 0.325 | 0.236 | -0.038 |
| Own values | 9.795 | 5.058 | 3.210 | 1.471 |
| Explained variance (%) | 40.814 | 21.077 | 13.378 | 6.132 |
| Accumulated variance (%) | 40.814 | 61.891 | 75.269 | 81.401 |
López-Gutiérrez et al., (2015) realized morphological comparison between wild and cultured populations of Opuntia atropes (Cactaceae) in Michoacán, Mexico; assessed morphological variables managed separating cultured and not cultured individuals by principal component.
In another research study realized by Martínez-Moreno et al., (2006), the three principal components were reported to show 84.16 % of the total variability existing in the individuals, which is obtained by measuring 18 variables. In another research study realized by Morales et al., (2016), the first two PC were reported to explain 91 % of the variance with nine variables. Both research studies are congruent with the percentage we reported with 81 % of cumulated variance with 24 assessed morphological variables.
Quantitative characters separated the twelve species into five groups according to the cluster analysis, with an average taxonomical distance coefficient of 0.28. In the first group seven species were located: E. runyonii, P. oviferum, E. elegans, E. perle, E. pumila, E. pulvinata, and E. agavoides, being the group where the highest number of species was gathered. General characteristics shared by the species from the first group are leaves in rosette, sagittal-elliptical shape of their leaves, and above all a distinct characteristic among them is the clusters of bunches of inflorescence and with a disposition of uniparous scorpioid cyme. The second group was integrated by E. derembergii and E. setosa, these species are similar in the shape of the rosette; however, E. setosa present tricomes in the leaves. The third group corresponds to S. clavatum, separated from Echeverias and Pachyphytum, which, although presenting similarity with them for sharing the morphology of leaves in rosette and shape of the flower, was distinguished by its small flowers (Figure 2).
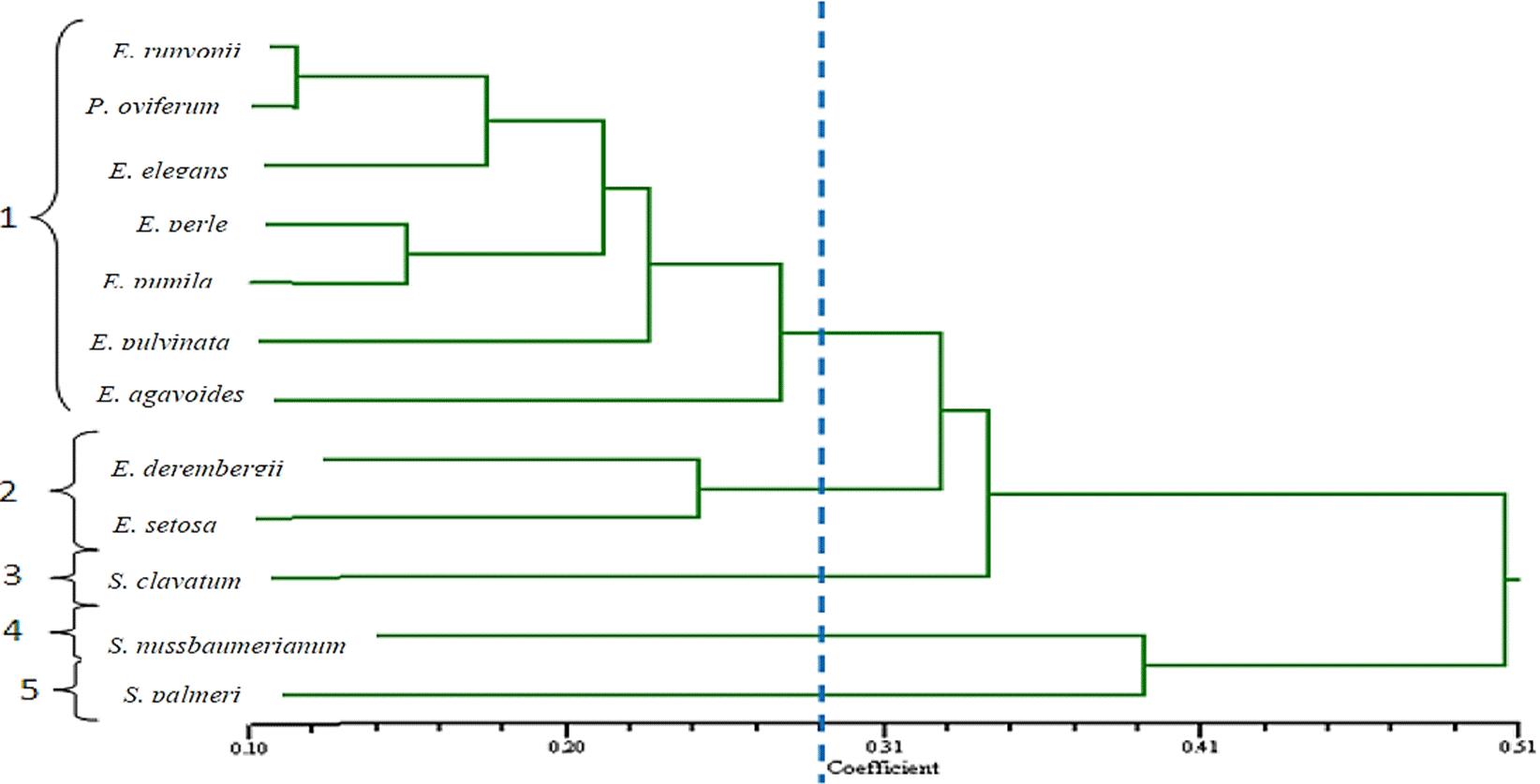
Figure 2 Fenogram of twelve species of the family Crassulaceae, constructed by the UPGMA method (average linkage) from 35 quantitative characters of the plant. The distance coefficient refers to the Average Taxonomic Distance.
The fourth group was formed by S. nussbaumerianum, this species clusters with S. palmeri (from the fifth group), at a taxonomic distance of 0.39; these are similar in growth habit, and inflorescence; these two species of Sedum are in turn bound with other ten species of Crassulaceae with an average taxonomical distance coefficient of 0.51, being these ones the farthest from the other species of Crassulaceae of the dendrogram (Figure 2).
As observed, P. oviferum clustered with six species of Echeveria, due to the fact that they share similar characteristic related to the shape of the leaves, color, size and inflorescence, but it should be said that its growth is not in rosette, but presents an ascendant ramification.
The most similar species according to the average taxonomical distance coefficient were E. runyonii with P. oviferum, in contrast, the most distinct species were S. nussbaumerianum and S. palmeri, which have an average taxonomical distance coefficient of 0.39 among them.
According to the results, it can be said that Crassulaceae is a very complex family, due to its easy hybridization and there could have crossbreeding not only among species of the same genus but among different genera as well, being the possible cause for which morphological similarity is shared among genera (Jones et al., 2010; Jimeno-Sevilla et al., 2013; Jimeno-Sevilla et al., 2014 and Rodríguez-Rojas et al., 2015). The current specific taxonomical classification might also be incorrect. In phylogenetic analysis of Crassulaceae, cladograms riddled with polytomies lacking of resolution has been found repeatedly (Acevedo-Rosas et al., 2004).
Molecular characterization
Twenty primers were selected for the molecular analysis, to present the highest number of amplified products, the brightest and the most defined bands. Primers generated fragments that varied from 231 to 4,774 pb.
Selected primers for the twelve species of the Crassulaceae family produced 594 RAPDs fragments, 99 % of them were polymorphic and allow to differentiate the twelve species with 76 markers. OPA-09, OPB-18 and SAP-03 primers did not generate markers fragments (Table 4).
Table 4 Initiators used to study twelve species of the Crassulaceae family.
| Primers | Amplified fragments | Polymorphism (%) | Markers fragments (pb) |
|---|---|---|---|
| OPA02 3′TGCGCCCTTC 5’ | 28 | 100 | 4171,4244, 1050 |
| OPA04 3′AATCGGGCTG 5’ | 38 | 100 | 3478, 3058, 1453, 641,585, 334, 240 |
| OPA07 3′GAAACGGGTG 5’ | 32 | 100 | 3211, 2634, 525 |
| OPA08 3′GTGACGTAGG 5’ | 32 | 100 | 1919, 1259, 1013, 801, 745, 395 |
| OPA09 3′ GGGTAACGCC 5’ | 22 | 100 | 0 |
| OPA10 3′GTGATCGCAG 5’ | 26 | 100 | 1858,1732,1618 |
| OPB01 5’ GTTTCGCTCC 3’ | 21 | 96 | 3095, 1229, 1066, 308 |
| OPB06 3′ TGCTCTGCCC 5’ | 25 | 96 | 2528,1956, 2526, 887, 1581, 373 |
| OPB07 3′GGTGACGCAG 5’ | 45 | 100 | 3135, 796, 231 |
| OPB08 5’ GTCCACACGG 3’ | 25 | 98 | 734, 694,595 |
| OPB10 3′CTGCTGGGAC 5’ | 39 | 100 | 2723, 2306, 2244,1441, 847, 1344, 267, 1303, 712, 441, 294 |
| OPB12 3′ CCTTGACGCA 5’ | 29 | 96 | 3275, 2506, 312 |
| OPB18 3′ CCACAGCAGT 5’ | 24 | 100 | 0 |
| OPB19 3′ ACCCCCGAAG 5’ | 32 | 100 | 4774,3172,1604, 4587, 1580, 1469, 636, 625, 395 |
| OPC08 3′ TGGACCGGTG 5’ | 30 | 100 | 2968, 1206, 1430 |
| OPC09 3′ CTCACCGTCC 5’ | 28 | 100 | 309 |
| OPC13 3′ AAGCCTCGTC 5’ | 24 | 96 | 2791 |
| OPC16 3′ TTTGCCCGGA 5’ | 26 | 100 | 788,353, 306, 282, 241 |
| SAP03 3′ TGGGACCTCC 5’ | 26 | 92 | 0 |
| SAP04 3′ GGAGCTACCT 5’ | 24 | 100 | 2935, 2682, 618, 333, 249 |
| Total | 594 | 99 | 76 |
Primers that generated the highest number of markers were OPB-10 and OPB-19, allowing to characterize the highest number of species. Primers that showed three markers were OPA-02, OPA-07, OPA-10, OPB-07, OPB-08, OPB-12 and OPC-08, those markers were presented in at least two species; OPC-13 and OPC-09 produced only one marker fragment each one and were useful to characterize one plant. No primer for itself allows to characterize all species. E. agavoides and E. pulvinata were those showing the highest presence of markers (Table 4).
Total and polymorphic RAPDs fragments obtained in the characterization of the twelve species of the Crassulaceae family, were higher than those obtained by Montalvo et al. (2010) who obtained a total of 97 bands, of which 62.8 % were polymorphic and on the other hand, in the present study, 594 bands were obtained, 99 % of which were polymorphic. The cluster analysis obtained by Montalvo et al. (2010) allow them to differentiate all genotypes; they could define the presence of a group made by individuals 1, 2 and 3, while genotype 4 was genetically farther. In our study, 6 different groups were separated in which groups did not behave according to their taxonomical classification. It has to be said that the study of Montalvo et al. (2010) was made up of 4 seeds of a same species, compared to the study realized, in which species from different genera were compared.
The similarity of the twelve species of the Crassulaceae family determined with the 594 fragments varied from 0.576 to 0.692; of the 66 obtained genetic similarities, six were lower than 0.6 and correspond to E. perle with E. pumilla, E. agavoides with E. elegans, E. agavoides with P. oviferum, E. pumilla with E. setosa, and E. pumila with S. palmeri; in contrast, the highest genetic similarity was obtained for E. runyonii with E. derembergii (0.692), E. setosa with S. palmeri (0.685), E. perle with E. agavoides (0.668), E. runyonii with E. perle (0.667), E. runyonii with S. palmeri (0.663), E. derembergii with P. oviferum (0.663), P. oviferum with S. nussbaumerianum (0.660). The highest genetic similarity was expected to be for species of the same genus, but it was not like that, various species of different genera were found within the pairs with the highest similarity, such as E. setosa with S. palmeri and P. oviferum with S. nussbaumerianum.
Associations of genetic markers by means of cluster analysis are considered to be very useful since they include a high number of traits and because it is a hierarchical classification which can be used to predict the threshold levels of similarity among species (Singh, 1999).
The obtained cluster analysis showed that, at a level of 0.65 of genetic distance, six clusters were formed: 1) E. runyonii, E. derembergii, P. oviferum, S. nussbaumerianum, being this one the cluster including more species; 2) E. setosa, S. palmeri, 3) E. elegans, E. pulvinata, 4) S. clavatum, 5) E. perle, E. agavoides, and 6) E. pumila, this last group being the most distinct from the rest, since it is the last incorporated to the group with a distance of similarity of 0.61 with the rest of the species (Figure 3).
The most similar species among themselves (with a coefficient of 0.62) were E. runyonii with E. derembergii, these ones were those that presented the lowest genetic distance. In contrast, the most distinct species to the rest is clearly E. pumilla, since it is the one that is incorporated to the group with the highest distance coefficient (0.6) (Figure 3).
The obtained clustering based on morphological traits has three similarities with the clustering generated, based on RAPDs fragments, E. elegans was clustered with E. pulvinata, as well as E. perle with E. agavoides, the third similarity was that Sedum clavatum was not clustered with any of the eleven species of the study.
Carrillo-Reyes et al. (2008) investigated Thompsonella and a group of Echeverias (Crassulaceae) and their phylogenetic relation, based on molecular and morphological traits, in which they realized a dendrogram with the molecular analysis, observing that species of a genus were clustered with those of another genus, similarly to what was obtained in the present research study, in which there is a discrepancy of genetic clustering related to the taxonomical classification, in both research studies, and they concluded that it was necessary to elucidate relations and generic limits of the Echeveria group and, moreover, in the Acre clade as a whole.
Acevedo-Rosas (2004) studied the molecular phylogeny of Graptopetalum (Crassulaceae) and concluded by saying that “this study highlights new interpretations of systematic relations within Crassulaceae and the role of geography, habitat, pollinators and others ecological factors might play in the conduction of the evolution of these succulent plants,” since in their study, clusters of distinct genera were presented as well as in our clusters of this study. In diverse studies, the wrong taxonomical classification of the Crassulaceae family has been discussed, this research study shows it is of great importance to realize a study gathering all the species of this family to elucidate the correct classification of the genera, by means of a program allowing to obtain molecular markers identifying and classifying them properly.
The total and polymorphic RAPDs fragments obtained in the characterization of twelve species of the Crassulaceae family, target of this research work, were higher than those obtained by García (2003) who employed genetic AFLP markers in Crassulaceae, in which they concluded that they obtained a total of 497 distinct bands with five used combinations, being polymorphic 375 bands (92.4 %). In another study realized by Montalvo et al. (2010) in plants of the Cactaceae family (Pilosocereus sp), the use of ISTR markers was reported, in which six combinations of ISTR oligonucleotides were used, and a total of 97 bands was obtained, of which 62.8 % were polymorphic. In contrast, in the present research work, 594 bands were obtained, of which 99 % were polymorphic. Carrillo-Reyes et al. (2008) realized a study to identify monophyletic groups to elucidate whether Altamiranoa and Villadia must be recognized as independent genera, arguing that Altamiranoa was divided into two groups, Subcampanulatae and Gamopetalae but afterward they saw the variability of the inflorescence in others genera of Crassulaceae and porposed the fusion of both taxa under the name of Villadia; posteriorly, due to a wrong interpretation of the slides of the nomenclatural types of Villadia and Thomsonella Britton & Rose, and then, they decided that the legitimate name for Thompsonella was Villadia and it was transferred to all species, from Villadia to Altamiranoa; but in 1940 they detected this mistake and Thompsonella was separated from Villadia and Altamiranoa.
In 1996, a superposition of traits was argued, based on the phylogeny of `t Hart, who proposed to include Altamiranoa in Sedum and to recognize Villadia, but some researchers still consider Altamiranoa an independent genus. As well, Mort et al. (2001) mention that the “Acre clade” includes a third part of Crassulaceae species, but is riddled with a series of unsolved relations.
Carrillo-Reyes et al. (2008) concluded that their combined analysis also present numerous unsolved relations and scarce groups with support. So they consider that, owing Sedum species are intermixed within those of all these genera, in addition of others being placed in independent groups, it is necessary to firstly define the limit and the composition of the rest of the taxa. Therefore, it may be said that Crassulaceae is a highly complex family; due to its easy hybridization and crossbreeding might have been not only among species of the same genus but also of different genera, being the answer to the genetic similarity they share with other genera.
The clustering of the morphological dendrogram of the twelve assessed species was not congruent with the molecular clustering, therefore there was no correspondence between the assessed morphological and molecular markers, which is coherent since they are different assessment methods and a wrong classification of species may likely exist, to confirm the aforementioned, a germplasm bank should be established to assess and correctly classify species, realize the assessment at least in two cycles, using morphological principal components that were highlighted in the present research work and run more primers allowing to properly discriminate species and allowing to locate them correctly.
Conclusions
Morphological and molecular markers detected genetic diversity among species. The obtained clustering, based on the morphological traits was congruent with the taxonomical classification since, in general, species of the same genus were clustered together. In contrast, in the molecular study, species of different genera were clustered together, indicating that there was no correspondence between the assessed morphological and molecular markers. The molecular analysis detected that species with higher genetic similarity were: E. runyonii and E. derembergii, indicating a higher genetic possibility of crossbreeding for future interspecific hybridizations.











 texto en
texto en 



