Introduction
Ziziphus obtusifolia (Hook. ex Torr. & A. Gray) is a plant that grows in a bush-like form, belonging to the Rhamnaceae family and it is commonly known as barchata. Research studies about this particular species are scarce.
Sharma et al. (2014), performed a review of the therapeutic properties of different species of the genera Ziziphus and found that their leaves, fruits, seeds, barks, and roots have the potential for the development of medications. In addition, it has been observed that there is an increasing demand for species of the genera Ziziphus, due to their potential application on human health.
On the other hand, it has been documented that some species of Ziziphus contain numerous therapeutic capacities like antibacterial agents with antioxidant and anti-cancer properties (Sharma et al., 2014). Moreover, for their high protein content, Bowers et al. (1993) and Ramirez et al. (2001) have proposed barchata (Ziziphus obstusifolia) as forage for ruminants.
Barchata is commonly found in northwestern Mexico, and Sonora is one of the endemic regions of this species, being an autochthonous plant; because of its accessibility, the ethnic population uses it as an aqueous infusion in alternative remedies for fighting cancer. Previous studies have proven that Ziziphus obstusifolia have antiproliferative and apoptotic activities (Ramirez et al., 2001; Molina et al., 2018). However, there are not enough studies that confirm the benefits and properties of this species on human health.
Therefore, the objective of this research work was to evaluate the antioxidant, antibacterial and antiviral capacity of the extracts of barchata branches.
Materials and methods
Reagents
AAPH (2-2´-azobis(2-methylpropionamidine dihydrochloride), DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and DMSO (Dimethyl sulfoxide), were obtained from Sigma Aldrich, Co. (St. Louis Missouri, USA). The Mueller-Hinton agar, and the rest of the reagents and solvents not specified were bought in J.T. Baker (BakerMallinckrod, Mexico).
Preparation of the extracts
Branches of barchata (Ziziphus obstusifolia) were obtained from southern Sonora, Mexico, during the period of May-December, 2016, and were identified by the herbarium of Universidad de Sonora (Specimen No. 20395).
For the preparation of the EtOH-ac extract, the method described by Sultana et al. (2009) was used. Branches of barchata (Ziziphus obstusifolia) were dried in the sun and ground with an industrial grinder. Ten grams of barchata powder were added to a 150-mililiter extractor solution (EtOH-ac) that consisted of a mixture of ethanol and acetic acid (95:5 % v/v) and was maintained in darkness for 48 h in maceration with intermittent shaking. After 12 h, the solution was filtered through a Whatman No. 1 filter paper and the extract was concentrated in a rotary evaporator (Yamato RE301-Japan) and was dissolved in dimethyl sulfoxide (DMSO) at 5 % for easier handling.
In order to produce aqueous infusions (Ac), 1 g of powder from the branches of barchata was placed in 100 mL of water and boiled for 3 minutes. The EtOH-ac extract was filtered and concentrated in the rotary evaporator; it was dissolved in water for its evaluation.
Antioxidant capacity
Inhibition of DPPH radical (2, 2-diphenyl-1-picrylhydrazyl)
The antioxidant capacity of the extracts was measured by means of the inhibition of the DPPH radical, according to Moein et al. (2010) with some adjustments. A 280 µL aliquot of the solution of the DPPH radical (0.025 mg/mL in methanol) was mixed with 20 µL of the extract, the reaction was kept for 30 min in complete darkness and the absorbance was read to a wavelength of 490 nm in a spectrophotometer Fluostar Omega (BMG Labtech, Chicago, Il, USA). Antioxidant activity was calculated using a calibration curve of trolox and results were expressed in mmol equivalent trolox/g of extract (mmol ET/ge).
Inhibition of ABTS radical (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
It was determined according to the technic described by Re et al. (1999). ABTS radical was prepared by dilution (19 mg/5 mL of distilled water) and a potassium persulfate solution (37.8 mg/mL of distilled water). Eighty-eight µL of prepared ABTS radical were added to the solution of potassium persulfate. The mixture was let to rest for 12-16 h at room temperature. From this solution, 500 µL were diluted in 30mL of ethanol for adjusting absorbance at 0.7 ± 0.02 units in a microplate reader at a wavelength of 750 nm. Finally, 295 µL of the radical and 5 µL of the extract were placed in the microplate, and initial measurements (Absi) and after 7 min (Absf) were performed. The antioxidant activity was calculated using a calibration curve of trolox and the results were expressed as mmol equivalent trolox/g of extract (mmol ET/ge).
Protective effect on human red blood cells
Hemolysis was induced by AAPH (2-2´- Azobis (2-methylpropionamidine dihydrochloride) radical according to methodology of Luy et al. (2010). Human red blood cells were washed 3 times with PBS (phosphate-buffered saline) (37mM of NaCl, 2,7 mM of KCI, 8 mM of Na2HPO4 and 2 mM of KH2PO4 P/V) at pH 7.4. Once washed, a suspension of red blood cells at 5% in PBS was prepared. For the test, 50 µL of the suspension of red blood cells, 50 µL of the extract, and 200 µL of AAPH radical were mixed, and the mixture was incubated at 37 °C in bain-marie with shaking (30 rpm) for 3h. A similar reaction mix was prepared without extract as a control (complete hemolysis). Once incubation finished, 1 mL of PBS was added, it was centrifuged at 3500 rpm for 10 min and the absorbance of the supernatant was measured in a microplate reader at 540 nm. The result was expressed in percentage of inhibition, which was calculated using the following formula: (Abs. Control- Abs. Final)/ (Abs. Control * 100).
Antiviral capacity
Bacteriophage propagation
Av08 bacteriophage was obtained from the ‘Laboratorio Nacional de Investigacion en Seguridad Alimentaria of the Centro de Investigacion en Alimentacion y Desarrollo (CIAD)’ in Culiacan, Sinaloa, Mexico. For the propagation of Av08 bacteriophage, the double-layer agar method described by Jamalludeen et al. (2009). Briefly, 100 µL of the bacteriophage were mixed with 1 mL of the bacterial host (E. coli 0157) in a tube containing 3 mL of tryptic soy broth (TSB) with 0.4 % agarose at 45-48 °C. The mixture was gently shaken and poured over Petri dishes with trypticase soy agar (TSA) medium; once the soft layer solidified, dishes were incubated for 18-24 hours at 37° C.
After incubation, 6 mL of SM buffer (MgSO4.7H2O 8 mM, NaCl 100mM, pork gelatin type A at 0.002 % P/V) were added to each dish and were shaken by oscillation for 3 hours. Buffer and soft layer were recovered by removal with a bacterial inoculation loop and were emptied in a 50 mL tube, then it was centrifuged at 10,000 x g for 15 minutes at 4° C in order to eliminate bacterial detritus and TSBagarose solid phase.
Supernatant was recovered and filtered through a nitrocellulose membrane (Whatman, USA) with pore size of 0.45 µm for its subsequent quantification and use.
Bacteriophage quantification
Decimal dilutions of the Av08 bacteriophage were prepared in SM buffer. Fifty µL from each dilution of the phage and 500 µL of the bacteria E. coli 0157 were placed in tubes containing 1.5 mL of TSB-agarose 0.4 %. The mixture was poured into Petri dishes with TSA corresponding to each dilution, and were left to solidify. Dishes were incubated at 37 °C for 24 hours and after this time, plates were read, counting the PlaqueForming Units (PFU/mL) of each dish and bacteriophage concentration was calculated with the following formula: PFU/mL= Number of plates [PFU]/(Dilution factor) (inoculated volume [mL])
Antiviral test
The antiviral effect of EtOH-ac and Ac extracts was measured against Av08 bacteriophage whose genetic material is dsDNA. Extracts were dissolved in 5 % of DMSO and were sterilized through a membrane filter with a pore size of 0.45 µm. Tested concentrations were 5 and 10 mg/ mL. An aliquot of 100 µL of the phage (1 X109 PFU/mL) was confronted with 3 mL of the extract. Three contact times were evaluated: 0, 30 and 60 min. At the end of each time, the reaction was stopped by adding a neutralizing solution of soy phosphatidylcholine, according to SCFI (1999). Next, decimal dilutions were prepared in SM buffer (10-2, 10-4, 10-6, 10-8, 10-10 y 10-12) and the phage was quantified employing the double-layer agar technique. Viral reduction was expressed as Log10 PFU/mL.
Antibacterial capacity
Bacterial strains and growth conditions
Microorganisms were obtained from the laboratory of Inocuidad Alimentaria del Centro de Investigacion e Innovacion en Biotecnologia, Agropecuaria y Ambiental (CIIBA) of the Instituto Tecnologico de Sonora (ITSON). Staphylococcus aureus (ATCC 65384) and Escherichia coli 0157 (ATCC 43890) bacteria were maintained in TSB with glycerol (20 %) at -40°C until test. With an inoculation loop, an aliquot of each bacteria was transferred into tubes containing 10mL of TSB and was incubated at 37°C for 24 hours for its use in inhibition tests.
Antimicrobial susceptibility test
Antimicrobial activity was evaluated by means of the disk diffusion test, applying the technique described by Andrews (2001). Petri dishes with Mueller Hinton agar were inoculated with 100 µL of the bacterial suspension (1 X 108 UFC/mL), which was homogenously distributed with sterile glass perlites. Separately, 40 µL of the corresponding extract (EtOH and Ac) were placed in sterile disks of paper filter (5mm in diameter, Whatman N° 1) which were placed on inoculated Petri dishes with each bacteria. Petri dishes were incubated at 37°C for 24 hours for further measurement of inhibition halos. Negative controls were included, which consisted of DMSO coated disks (5 %).
Statistical analysis
The evaluation of antioxidant activity measured by inhibition of radicals (DPPH, ABTS and AAPH), in addition to the antimicrobial one was realized by means of a oneway variance analysis (ANOVA) with independent assays and totally randomized with three repetitions, considering the “extract” factor with two levels (EtOH-ac and Ac).
In the statistical analysis of antiviral activity against Av08 phage data, a totally randomized three-factors design was used. The factors were “extract” with two levels (EtOHac and Ac); “concentration” with two levels (5 and 10 mg/ mL), and “contact time” with three levels (0, 30, and 60 min). All the experiments were performed in duplicates with two repetitions each. All data were analyzed using Statgraphics version 15 statistical software through ANOVA and α=0.05.
Results and discussion
Antioxidant capacity of the extracts by means of DPPH radical
In this research study, the capacity of EtOHac and Ac extracts of barchata to inhibit DPPH radical was evaluated. Radical inhibition was shown in Figure 1. Inhibition values of 25.03 ± 3.73 mmol ET/ge y 23.06 ± 0.24 mmol ET/ge can be observed for Ac and EtOH-ac extracts, respectively. Results showed that the antioxidant capacity of the extracts vary according to their chemical nature and to the solvents used in the extraction of substances with biological activity.
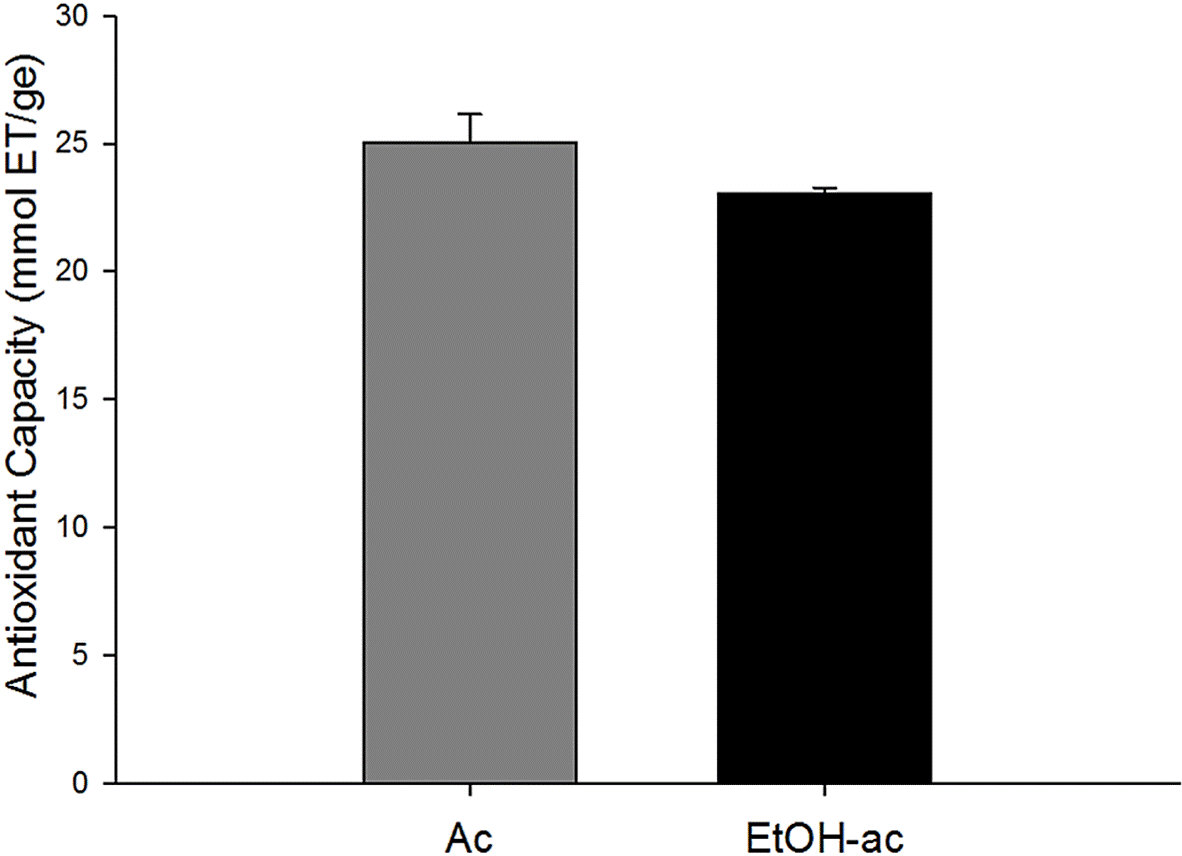
Figure 1 Antioxidant capacity by DPPH assay of Ac and EtOH-ac extracts from barchata (Zizhupus obtusifolia). The values are the mean ± standard deviation (n= 3). ET, Equivalent Trolox (homolog of vitamin C) ge, gram of extract.
The results obtained in this research study were in agreement with those described by Kang et al. (2003), where they performed assays on antioxidant capacity of natural extracts with different solvents, not only polar but also non-polar, and concluded that the antioxidant capacity was affected by the solvent used.
During the present work, research studies that documented the antioxidant features of Ziziphus obtusifolia, measured by inhibition of synthetic radicals like DPPH, were not found. However, Dias et al. (2013), observed that ethanol extracts of species of Ziziphus have aglycones that present capacities of free radical reduction up to 25 %, and reported that the extracts presenting a high concentration of glycosylated saponins reduced their antioxidant activity. Similarly, Navas & Carrasquero (2012) studied the antioxidant activity provided by ether extracts of Ziziphus mauritiana in refined soybean oil at high temperatures, observing a high protection from oil by this extract, and they attributed the activity to the presence of bisphenols, flavonoids, and tannins.
Antioxidant capacity of the extracts by means of radical ABTS
In this work, the capacity of the extracts to inhibit the ABTS cationic radical (2,2’-azino-bis(3ethylbenzothiazoline-6-sulfonic acid). Figure 2 shows the antioxidant capacity for the extracts by means of reduction of this radical, where the values 17.41 ± 0.01 mmol ET/ge and 23.35 ± 0.02 mmol ET/ge for the extracts Ac and EtOH-ac were obtained; respectively. As well, it was observed that the acidified ethanol extracts showed higher capacity to inhibit this radical. Dorta et al. (2013) observed that the acid systems were more effective at recovering bioactive compounds, and that they possibly could favor bioavailability and hydrolysis of N-glycosylated compounds. Barchata contains nitrogenous compounds such as glutamic acid and aspartic acid (Moran et al., 2014), and according to Wu (2009) these active substances present antioxidant activity.
Antioxidant capacity by means of hemolysis with AAPH radical
Figure 3 shows the protective capacity of Ac and EtOH-ac extracts on human red blood cells, using AAPH radical. The graph indicates that the values for the percentage of inhibition of hemolysis were 46.3 % and 36.8 % for Ac and EtOh-ac extracts, respectively.

Figure 3 Antioxidant capacity by the assay of % inhibition of hemolysis induced by AAPH of acidified aqueous and ethanol extracts of barchata (Ziziphus obtusifolia).
Recently, biological models like human red blood cells have been used, with the purpose of determining the antioxidant capacity of diverse bioactive substances on cell lipid peroxidation (Quihui et al., 2017). This method is based on the induction of hemolysis generated by AAPH radical (2,2’-Aazobis (2-amidinopropane) dihydrochloride). This radical causes the release of iron from the hemoglobin, acting as a pro-oxidant. The release of iron from red blood cells may increase the pro-oxidant effect of hydroperoxides coming from the reaction of oxygen and AAPH (Velioglu et al., 1998).
No research works evaluating the capacity of barchata extracts to protect human red blood cells using AAPH radical were found. Similarly, no antecedents on Ziziphus genus, assessing this property were found. For the purposes of this research, results were compared with similar studies.
Magalhaes et al. (2009) analyzed the percentage of inhibition of hemolysis of methanol extracts from the peel and pulp of quince and reported values of 70 and 75 %, respectively. On the other hand, it was demonstrated that compounds like gallic acid, ellagic acid, chlorogenic acid, quercetin, catechin, rutin, carotenoids, among others, have a protective effect against the hemolysis induced on human red blood cells up to 99 % (Chirinos et al., 2008; Hapner et al., 2010). It has been reported that the extracts of leaves of the Toona Sinensis plant present inhibition values from 75 to 85 % (Hseu et al., 2008).
In general, it was observed that diverse reports show values of the order of 30 % to 40% superior to those found in our research. This divergence could be explained based on the diversity of techniques used to obtain the extracts, or, to their composition. Previous reports show that species of Ziziphus contain saponins (Ribeiro et al., 2013), which can cause hemolysis in red blood cells (Martinez, 2001), suggesting a certain cytotoxicity.
Studies realized by Molina-Romo et al. (2018) argued that Ziziphus obstusifolia has an antiproliferative effect, therefore its potentialities against tumor and cancer cells cannot be completely discarded.
Antibacterial capacity
Antibacterial activity in EtOH-ac and Ac extracts is presented in Table 1, measured by inhibition halos. Values for concentrations of 5 and 10 mg/mL, respectively, were the following: E. coli 0157 bacteria, showed values from 7.83 to 10.16 mm in diameter in EtOH-ac extract, while in Ac extract, inhibition values ranged from 7.83 to 8.00 mm. For Staphylococcus auerus bacteria, EtOH-ac extract showed results from 8.00 to 9.00 mm in diameter and from 8.00 to 9.06 mm in Ac extract.
Table I Antibacterial capacity of Ziziphus obtusifolia extracts. Each value represents the mean of three data.
| Bacterial inhibition | |||
|---|---|---|---|
| Extraction | Concentration mg/mL | E. coli O157 mm | S. aureus mm |
| EtOH-ac | 5 | 7.83±0.1 | 8.00±0.2 |
| 10 | 10.16±0.5 | 9.00±0.5 | |
| Ac | 5 | 7.83±0.8 | 8.00±0.5 |
| 10 | 8.00±0.0 | 9.06±0.9 | |
| Control: DMSO 5 % | 5 | SA | SA |
| 10 | SA | SA | |
NA: No activity
A similar study on Zanthoxylum extracts mentions inhibition halos obtained from some antibiotics like tetracycline with 9.00 mm for S. auerus and 10.00 mm for E. coli; anthracycline with 12.00 mm for S. auerus and 10.00 mm for E. coli; and kanamycin with 9.00 mm for S. auerus and E. coli (Patiño et al., 2011). Generally, the results obtained from the two types of extracts were very similar to the antibiotics; therefore they could be considered as good antibacterial agents against the studied bacteria, in addition, these results were promising for continuing with more specific antimicrobial studies.
On the other hand, it was also observed that at increasing extract concentration , the inhibitory activity against the assessed strains increased as well, indicating that the antibacterial activity of the extracts depends on the concentration.
Barchata contains bioactive substances such as valine or leucine amino acids (Moran et al., 2014). These compounds are classified as bacteriocins, which are effective against important pathogenic microorganisms like Listeria monocytogenes, Staphylococcus auerus, Escherichia coli, and Salmonella spp. (Beristain et al., 2012); therefore these substances might contribute to the inhibition of the pathogenic bacteria used in this study.
On the other hand, in one of the studies realized on species of which this plant belongs, certain substances like tannins, saponins, resins, polyphenols, and cardiac glycoside were detected (Abalaka, 2010). As well, it has been reported that these compounds have antimicrobial activity (Moran et al., 2014), allowing, as well, to suggest that these substances contribute to the inhibition of the microorganisms used in this study, which are pathogenic organisms of interest in public health. Moreover, it is known that in developing countries where medicine has a high economic value, research on antibacterial activities of ethnomedicinal plants keeps being a necessity to fight against pathogenic microorganisms, which represents an opportunity area to treat diseases originated by these organisms.
Antiviral capacity
Ac and EtOH-ac extracts were applied to Av08 bacteriophage in order to determine viral survival rate and the effective dose at the required time, thus observing the reduction of the virus, caused by the effect of the extract.
Logarithmic reductions can be observed in Figure 4 for EtOH-ac extracts and in Figure 5 for Ac extracts, which show the reductions of the virus expressed in Log10 PFU/ mL for three contact times: 0, 30 and 60 minutes, using the two concentrations of 5 and 10 mg/mL. Similarly, it can be observed that in both extracts at time 0 minutes, there was no significant reduction in comparison to the control value (P < 0,05), nevertheless, at 30 minutes of contact time, a significant reduction was observed.
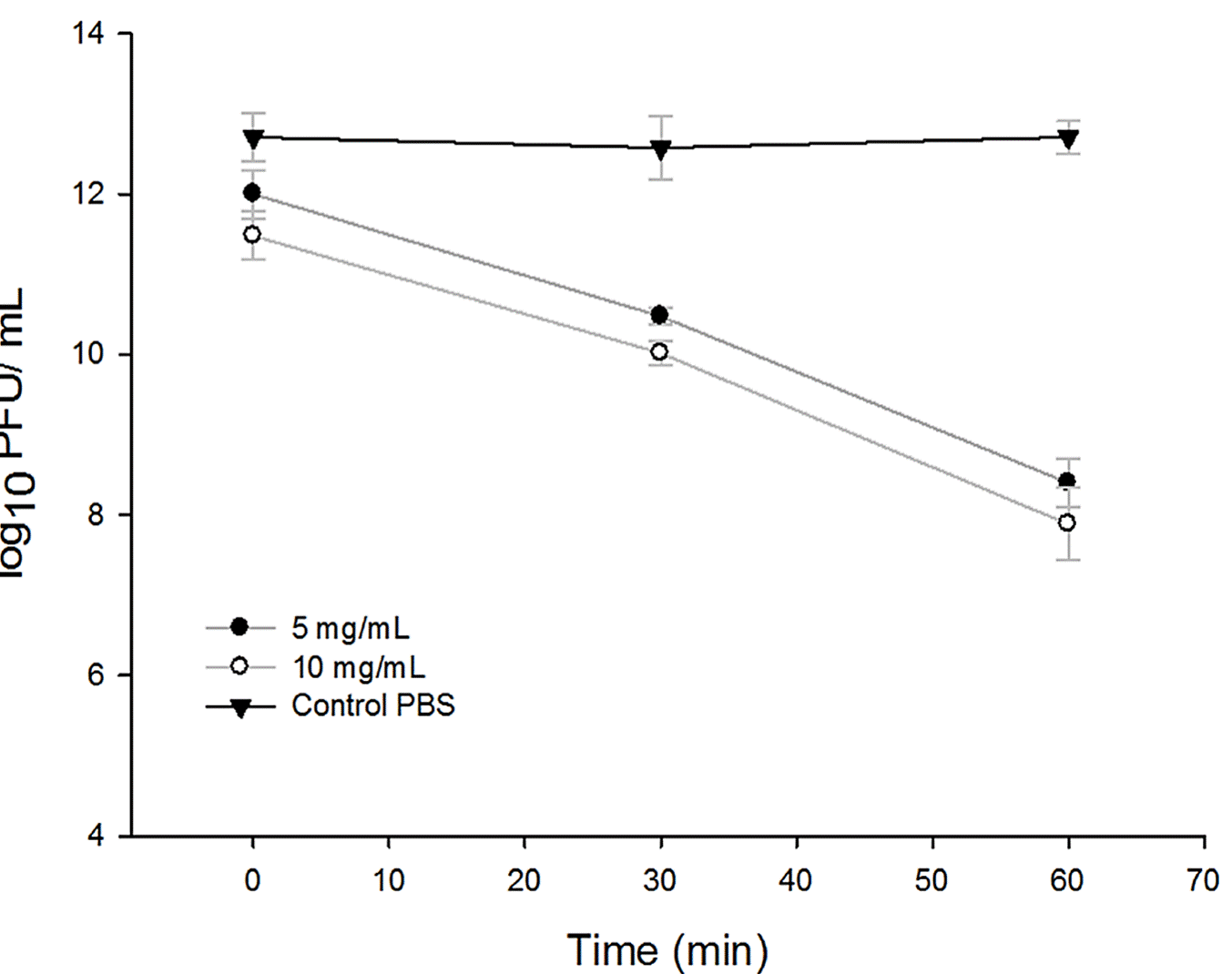
Figure 4 Antiviral activity of the EtOH-ac extracts of Ziziphus obtusifolia measured by the reduction in Log10 PFU / mL for the contact times 0, 30 and 60 minutes at two concentrations 5 mg /mL and 10 mg/mL.

Figure 5 Antiviral activity of the aqueous extracts of Ziziphus obtusifolia measured by the reduction in Log10 PFU / mL for contact times 0, 30 and 60 minutes at two concentrations 5 mg / mL and 10 mg / mL
On the other hand, in EtOH-ac extract in the first 30 minutes of contact with Av08 phage, the concentration of virus showed reductions of 1.5-1.8 Log10 PFU/mL for concentrations of 5 and 10 mg/mL, respectively. At maximum contact times of the extract with the phage, a reduction of 4.30 Log10 PFU/ mL was observed at a contact time of 60 minutes for the concentration of 5 mg/mL, and at the concentration of 10 mg/mL at the same time, the concentration of virus showed a reduction of 4.82 Log10 PFU/mL (Figure 4).
For Ac extract in both concentrations of 5 and 10 mg/mL, during the first 30 minutes, no significant reductions were observed, achieving values of reduction of the order of 0.70 Log10 PFU/mL, and it was only until 60 minutes of contact that the concentration of virus decreased 1.50 logarithms at the concentration of 5 mg/mL. Nonetheless, the highest shown reduction was at the concentration of 10 mg/mL, decreasing up to 2 logarithms, possibly due to the depletion and reduction of the bioavailability of the active metabolites or compounds after 30 min of contact time with the extracts.
DNA Av08 bacteriophage used in this study was previously isolated from poultry feces. Some studies demonstrated that bacteriophages isolated from poultry are part of the environmental microbiota, which demonstrates the natural occurrence of phages in the intestinal tract. Similarly, with the afore-mentioned, its use as enteroviral model is justified (Lopez et al., 2012).
There are no specific studies in Ziziphus oftusifolia that demonstrate that the compounds are responsible for the biological activities in enterovirus; However, in a study of amino acids determination, it was found that barchata contains alanine, valine, and leucine (Moran et al., 2014), which are substances to which antimicrobial capacities pertaining to some plants are attributed (Beristain et al., 2012). Another study realized by Eun-Hye et al. in 2015, found that the betulinic acids derived from Ziziphus jujuba showed antiviral activity against A/ PR/8 influenza virus induced in rats. Moreover, a study where the antiviral activity of medicinal plants was assessed against Hepatitis B virus (VHB) in cellular lines found that Eucalyptus spp. extracts showed the highest antiviral activity in the studied plants, where phenolic compounds were observed to be responsible for this inhibitory activity and, in Ziziphus, the presence of phenolic substances has already been documented (Gonzalez et al., 2006). That is why the antiviral activity of these compounds is not discarded.











 texto en
texto en 



