Introduction
Maize crop (Zea mays L.) is the fourth most important crop around the world. In Mexico, approximately 70 % of the total production comes from eight states: Chiapas, Guerrero, Jalisco, Mexico, Michoacan, Puebla, Sinaloa and Veracruz (Juárez, 2013). Viruses are important agricultural pathogens causing significant economic losses on yield and quality reduction depending on the geographic region (Ali & Yan, 2012). They can occur in single or mixed infections affecting photosynthetic processes and, some of them, also interfere with plant development. The number of reports on mixed infections has increased. Although these increased reports present new problems, they are also providing valuable information for disease control strategies (Rentería et al., 2011).
Maize dwarf mosaic virus (MDMV), transmitted by aphids, was the first virus reported to cause significant yield reduction on corn crops in the United States. This virus is a member of the Potyvirus genus and Potyviridae family, and remains among the most harmful viruses causing diseases of maize in various regions of the world, mostly in some Asian and European countries (Gell et al., 2010). More recently, infections of Maize chlorotic mottle virus (MCMV) (genus Machlomovirus, family Tombusviridae), transmitted by thrips, have been important in some countries (Silva-Rosales et al., 2015; Wangai et al., 2012). Other viruses such as Maize necrotic streak virus (MNeSV), Maize rayado fino virus (MRFV) and Maize fine streak virus (MFSV) also cause corn diseases. In Central and South America, MRFV causes the “rayado fino” or “achaparramiento” of maize, one of the most known diseases in corn (Vasquez & Mora, 2007). In Mexico, several viruses that infect maize have been reported, such as MRFV, MCMV, Maize mosaic Nucleorhabdovirus (MMV), (genus Nucleorhabdovirus, family Rhabdoviridae), transmitted by leafhoppers (Dalbulus maidis); and Sugarcane mosaic virus (SCMV), (genus Potyvirus, family Potyviridae), transmitted by aphids (Carrera et al., 1989; Espejel et al., 2006; Gordon el al., 1985). Other viruses, whose presence has not been reported so far are: Maize stripe virus (MSpV), (genus Tenuivirus, unassigned family) transmitted by leafhoppers (Peregrinus maidis); Brome mosaic virus (BMV), (genus Bromovirus, family Bromoviridae), transmitted by aphids and nematodes (Xiphinema sp.); and Maize white line mosaic virus (MWLMV), (genus Aureusvirus, family Tombusviridae), transmitted by plasmodiophora.
Corn stunt disease, caused by Spiroplasma kunkelii (usually referred as corn stunt spiroplasma or CSS), transmitted by leafhoppers (Dalbulus maidis) can be a devastating disease in the American continent, particularly in areas with high temperatures and low relative humidity. In the field, CSS can co-infect maize plants together with MRFV, causing up to 40-50 % of crop losses (Gordon et al., 1985). CSS is one of the most important pathogens affecting maize productivity in Mexico and little is known about its distribution and incidence in this country (Alcántara-Mendoza et al., 2010). Also, in maize plants the “achaparramiento del maíz,” caused by three pathogens: Spiroplasma kunkelii, MDMV and MRFV have been detected, as well as the maize corn lethal necrosis disease (MLN or MLND or CLN), which is a result of mixed infections of MCMV, SCMV and/or other Potyvirus. Potyviruses seem to be the most important worldwide, due to their economic impact (Redinbaugh & Pratt, 2009). Their incidence in combination with other viruses has not been fully addressed. However, a recent report from Ethiopia, pinpoints their importance with the presence of MLN and its negative effects on the maize sector (Mahuku et al., 2015).
Considering that interactions among viruses are widespread and crucial in viral evolution and also that crop damages, due to plant pathogens, may be prevented or at least reduced once they have been identified, we tested maize samples collected from Veracruz, Mexico. The objectives of this work were to identify the viruses that were present in this area and to determine if they can co-infect maize plants to the same extent and distribution in this corn-producer State.
Materials and Methods
Sample collection
Maize samples were collected from four municipalities in Veracruz: Cosoleacaque, Paso de Ovejas, Medellin and Tlalixcoyan during 2006-2007 year survey (Table 1). Adult maize plantations were sampled at the onset of inflorescence emergence. A total of 228 leaf samples from 28 identified genotypes were collected, some showing putative viral symptoms such as chlorotic spots, yellowing, red streaks, necrosis and plant stunting. Midribs were removed from the collected leaves and the samples were placed in plastic bags inside an ice-filled box and further stored at -70 °C until ready for testing.
DAS-ELISA assays
The double-antibody sandwich indirect method of the enzyme-linked immunosorbent assay (DASELISA) was performed using commercial kits which included reagent sets as well as positive and negative controls (Agdia, PathoScreen, Elkhart, IN). For these tests, 100 μl of crude leaf extracts (in 1:10 w/v extraction buffer) were used. The color reaction was developed using p-nitrophenyl phosphate (PNP), and the absorbance was read at 405 nm in a microtiter plate reader (Ultramark Bio-Rad). Maize leaf samples were tested against seven viruses to detect putative MDMV, SCMV, MCMV, MSpV, BMV, MMV and MWLMV Mexican strains. Also, CSS was surveyed using the same procedure. Virus positive and negative controls were included in all tests. Samples were positive when the absorbance value was at least three times greater than that of the negative controls.
Northern blot analysis
In the case of ELISA tests for SCMV and MDMV, cross reaction can occur using antibodies, as indicated by the manufacturer (Agdia Inc) and by our own previous experience. MDMV antibodies cross react with SCMV and not otherwise. In order to alleviate any possible ambiguity of a cross reaction, we hybridized, through Northern blots, RNAs from all the samples with a CP probe of a previously reported isolate from Veracruz SCMV-Mx (Espejel et al., 2006). Northern blots were performed as previously reported (Lindbo & Dougherty, 1992). To this, total RNA was isolated from leaf tissue, using LiCl and ten micrograms were separated by electrophoresis using 1.2 % formaldehyde denaturing agarose gel, transferred to Hybond N+ nylon membranes (Amersham) and cross-linked by exposure to UV radiation. Hybridization was performed using in-house prepared pre-hybridization and hybridization buffers and riboprobes labeled with [α-32P] dCTP (Promega, In vitro transcription system). Hybridization was carried out 65 °C with the nylon membrane in a series of SSC (0.1 % sodium dodecyl sulfate and NaCl and 0.015 M sodium citrate solutions), with increasing stringency. We detected the hybridizing signal in a Storm 860 apparatus using the ImageQuant version 1.1 BioRad. We recorded a signal of 800 bp size band as positive for viral presence. Healthy plants were used as negative controls and a previously identified infected plant was used as positive control. Sequencing of the CP of selected samples was done by conventional Sanger sequencing of the RT-PCR amplified products using our institutional service.
Maize Genotypes identification
Genotypes identification was made by The International Maize and Wheat Improvement Center (CIMMYT) and the Regional Maize Program in Central America, who have developed some of these maize tropical hybrids (Jeffers et al., 2004), as well as the National Institute for Forestry, Agricultural and Livestock Research (INIFAP) who developed the H-518, H-520 and H-513 (Sierra et al., 2004).
Results and Discussion
Identification of virus and spiroplasma
A total of 228 maize samples were collected from the sampled municipalities (Figure 1 and Table 1) during the two-year survey. In all samples, we found 11 discrepancies between DAS-ELISA analyses and Northern hybridization for SCMV representing a 4.8 % variability between the two methods. These discrepancies are explained by the fact that the two techniques detect different viral components (coat protein in ELISA and genomic RNA in Northern blots). Sequencing the SCMV-CP cistron was done to confirm the nature of SCMV in the DAS-ELISA positive samples (Data no shown).
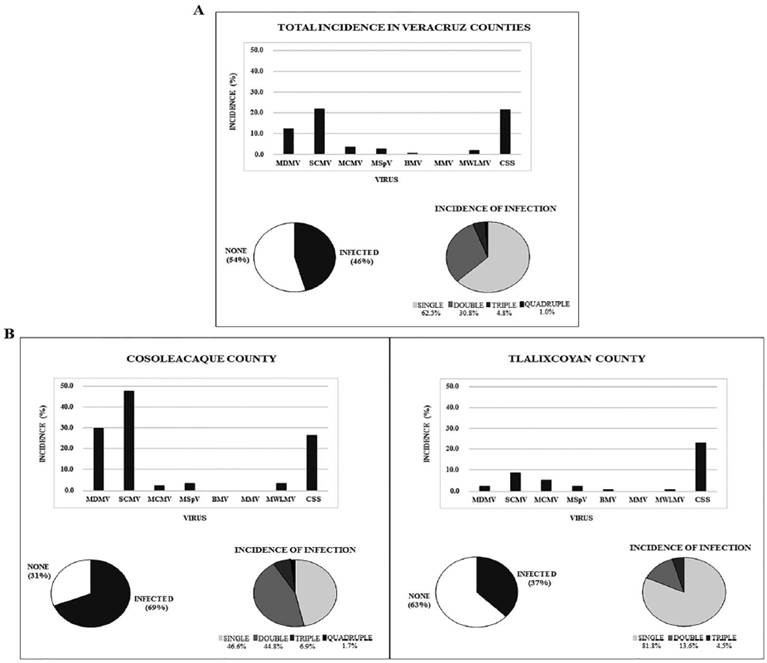
Figure 1 Relative abundance of six viruses and spiroplasma. A) Results of the sum of all positive samples from all municipalities. B) Incidence in Cosoleacaque and Tlalixcoyan. In all cases, circles represent the distribution of infected and non-infected plants and types of mixed infections.
Virus identification and their abundance in Veracruz regions showed that about 46 % (105/228) of the tested plants harbored at least one of the viruses and/or spiroplasma (Figure 1A). Laboratory testing of all samples collected indicated that their overall abundance of single, double, triple and quadruple infections was 62.5, 30.8, 4.8 and 1.0 %, respectively (Figure 1A). MDMV, SCMV, MCMV, MSpV, BMV and MWLMV were detected in single or mixed infections. MMV was absent whereas SCMV was the most common virus infecting 21.9 % (50/228) of the samples analyzed. CSS infection had similar abundances as SCMV with 49/228 (21.5 %). Together they were found in double, triple and quadruple infections (Table 2). CSS was followed in frequency by MDMV, MCMV, MsPV, MWLMV and BMV present in 12, 3.5, 2.6, 1.8 % and 0.4 %, respectively in the samples (Figure 1A). In the two-year survey, MDMV+SCMV was the most frequent double-infection combination in samples from the Cosoleacaque municipality as depicted on a pie chart (Figure 1B) and the most common triple infection was MDMV+SCMV+CSS. Only one sample was infected with the four-virus combination MDMV+SCMV+MCMV+MWLMV (Table 2).
Table 2 Maize viruses in single or mixed infections
| Cosoleacaque county | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. of
samples with virus |
MDMV | SCMV | MCMV | MSpV | BMV | MMV | MWLMV | CSS |
| 20 | + | + | - | - | - | - | - | - |
| 2 | - | - | - | + | - | - | - | - |
| 14 | - | - | - | - | - | - | - | + |
| 1 | + | - | - | - | - | - | + | + |
| 3 | + | + | - | - | - | - | - | + |
| 10 | - | + | - | - | - | - | - | - |
| 1 | + | + | + | - | - | - | + | - |
| 4 | - | + | - | - | - | - | - | + |
| 1 | - | + | - | - | - | - | + | - |
| 1 | - | + | - | + | - | - | - | - |
| 1 | - | - | + | - | - | - | - | - |
| Tlalixcoyan municipality | ||||||||
| 4 | - | - | + | - | - | - | - | - |
| 23 | - | - | - | - | - | - | - | + |
| 3 | + | + | - | - | - | - | - | - |
| 1 | - | - | + | - | - | - | - | + |
| 1 | - | - | - | - | + | - | - | - |
| 1 | - | - | - | + | - | - | - | + |
| 2 | - | - | - | + | - | - | - | - |
| 6 | - | + | - | - | - | - | - | - |
| 1 | + | + | - | - | - | - | - | + |
| 1 | - | + | + | - | - | - | + | - |
| 1 | - | + | - | - | - | - | - | + |
| Medellin and Paso de Ovejas municipality | ||||||||
| 2 | - | - | - | - | - | - | - | + |
Distribution and abundance of viruses, and spiroplasma
In the Cosoleacaque municipality, a total of 84 samples were analyzed (Table 1) and about 69 % of them had single, double, triple and quadruple infections with a corresponding 31 % without viruses nor spiroplasma. The most predominant type of infection, represented on a pie chart, in this region, was the single infection with SCMV (48 % abundance), followed by the single infections as MDMV (30 %) and CSS (26 %), on 14 samples (Figure 1B). Only one plant was infected simultaneously with four viruses and triple infections were detected in four samples (Table 2). In these cases, MDMV and CSS prevailed. Infections with CSS spiroplasma were detected in single or mixed infections with MDMV, SCMV and MWLMV, but not with MCMV, MSpV and BMV. The viruses BMV and MMV were absent in Cosoleacaque (Figure 1B). MCMV was the virus with fewer occurrence in Cosoleacaque (2.4 %), being almost 20 times less than SCMV. The most common and widespread virus in the areas surveyed was SCMV, being Cosoleacaque the municipality with the most incidence of this virus. Also, this municipality was the location that registered the highest percentages of infected samples with viruses and spiroplasma infections.
In the Medellin municipality, we only collected five samples from maize fields. Viruses were not detected in samples from this region and only one plant was positive for CSS infection. In Paso de Ovejas, 21 samples from four regions were analyzed with similar results as those from Medellin, no viruses were detected but one sample had CSS infection. In the Tlalixcoyan municipality, 118 samples were collected (Table 1). In this municipality 63 % of the plants analyzed were free of virus or CSS but 37 % (44/118) of the samples were infected (Figure 1B). Of these, single infection was predominant (81.8 %), with CSS prevailing. SCMV was also present in 10 positive samples (8.5 % abundance on Figure 1B) being the most common virus in this county. In triple infections, SCMV virus was prevalent. Infection with CSS spiroplasma was present in 23 % of the samples. In Tlalixcoyan, CSS was present in single or mixed infection with diverse viruses such as MDMV, SCMV, MCMV and MSpV but not with BMV, MMV nor MWLMV (Table 2). However, plants infected with BMV in a single or mixed infection were detected, in contrast to results obtained from Cosoleacaque, Medellin and Paso de Ovejas. MMV virus was not detected in any sample analyzed. MCMV virus had a 5 % abundance followed by MDMV and MSpV with 2.5 %. Less occurring viruses in Tlalixcoyan were BMV and MWLMV, with 0.8 % (Figure 1B). The absence of MMV and lower occurrence of MSpV could be explained to a lower presence of the leafhopper (Peregrinus maidis) vector. In fact, we only found one report of the presence of MMV and its vector in Tabasco in 1984 (Rocha-Pena et al., 1984). However, we did not survey for the distribution of this vector in the areas of study. The higher amount of viral incidence of SCMV and MDMV could be due to differences in temperatures between Cosoleacaque and Tlalixcoyan municipalities registered by Conagua Services (Weather Service in Mexico), (Conagua 2018). Indeed, changes in temperature allow differences in populations of insect vectors like aphids (Yañez-Morales & Peña-Martinez 1991). On the contrary, we would assume that cicadellidae population (leafhoppers) might not be fluctuating as much as that of the aphids, as they have a wide oviposition temperature range (15-40 °C) (Van Nieuwenhove et al., 2016), guaranteeing their similar population levels with a concomitant prevalence of CSS in both locations. The results of the surveys showed that MDMV, SCMV, MCMV, MSpV, BMV and MWLMV were viruses that infected maize grown in the Veracruz regions. In Mexico, SCMV was first reported in maize in 2006. This virus can infect different crops (e.g., maize, sorghum and sugarcane) causing symptoms such as chlorosis, mosaics and stunting (Espejel et al., 2006). During the surveys reported here, we observed maize plants with the characteristic symptoms of a SCMV infection, and in our diagnosis assays, we confirmed the prevalence of this virus. In the early phase of an epidemic, one individual becomes infected with a single virus at a time; when the epidemic continues, an increase in the density of virus population occurs and raises the possibilities for mixed viral infections (Lamichhane & Venturi, 2015; Xia et al., 2016). SCMV and all other viruses analyzed in this study were found in single or mixed infections. During our analysis, we detected the occurrence of MCMV co-infecting maize plants with SCMV and MWMLV in a triple infection; and in a quadruple infection with MDMV, SCMV and MWLMV. MCMV was also in mixed infections not only with other viruses, but also with CSS. At the present, we are not aware of any study addressing the interaction of viruses with CSS and their effect on disease severity. In this study, MDMV was also found in the areas surveyed with 12 % abundance. Mixed infections were previously reported involving MDMV with MRFV, SCMV or MCMV viruses (Redinbaugh & Pratt, 2009) but not with MWLMV as we report here, or with SCMV, MCMV and MWLMV in a double, triple and quadruple infections. During this survey, we detected MSpV, BMV and MWLMV in single or mixed infections. These viruses have been reported in several countries (Sharma & Misra, 2011); however, in Mexico, there are no reports of their presence. In this study, we found that these viruses are present infecting maize fields, not only in a single manner, but also in combination with other viruses and spiroplasma.
Corn stunt disease (CSD) is considered one of the most economically significant diseases of maize in the United States, Mexico and Central and South America. The severe stunt maize disease can be caused by the interaction of three pathogens: MRFV, CSS and Maize bushy stunt spiroplasma (Bradfute, Tsai & Gordon, 1981). Previous studies about presence of CSS, revealed their high prevalence and wide distribution in Mexico and confirm that maize plants are often infected with this phytopathogen (Alcántara-Mendoza et al., 2010; Gordon et al., 1985). CSS was identified in some samples from all areas analyzed. This spiroplasma was found in co-infections with MDMV, SCMV, MCMV, MSpV and MWLMV in a double, triple and quadruple infection (Table 2).
Maize genotypes and virus incidence
In this study, we try to correlate the genotypes of maize samples with the incidence of infection. Among our samples, we found 28 maize genotypes (Table 3). Twenty-eight maize genotypes were identified among the samples collected and 22 of them showed single or mixed infection with viruses and/or spiroplasma. Only one genotype 24. 1/33 A-7573 /Tornado showed a quadruple infection and we therefore cannot claim this as a susceptible genotype to a multiple viral infection. The genotype mostly collected was A-7573 including 117 samples and distributed in Cosoleacaque, Paso de Ovejas and Tlalixcoyan. Out of the total of these genotype samples, 38 % (44/117) harbored at least one virus or spiroplasma. Criollo and VS-536 genotypes were identified in 13 and 11 samples respectively (Table 3). In both cases, 9 samples were positive to virus and/or spiroplasma diagnosis. Forty-nine samples belonged to diverse genotypes distributed in all communities. Thirty-eight samples did not correspond any of the genotypes analyzed. These unknown genotypes samples were distributed along all communities and 29 % (11/38) showed single or mixed infection by viruses and/or spiroplasma. In the present study, the prevailing genotype in the survey was A-7573, which had previously been identified as susceptible to corn stunt disease (Sierra-Macías et al., 2010). On the other hand, four samples were identified as H520. It was reported that the genotypes with greater tolerance to the stunt maize disease are H-520, H-513, H-518 and C343, representing an alternative in commercial sowings for maize production in Veracruz state, with a possible resistance to maize stunt disease (Sierra-Macías et al., 2010).
Table 3 Maize genotypes in Veracruz Regions
| Genotipo | No. de muestras |
Comunidad | Infección Positiva |
|---|---|---|---|
| 24. 1/33 A-7573 /Tornado | 1 | Cosoleacaque | 1/1 |
| 30. 10/6 criollo /VS-536 | 1 | Cosoleacaque | 1/1 |
| 40. 6/33 VS-536 /Tornado | 1 | Cosoleacaque | 1/1 |
| 45. 6/10 VS-536 / criollo | 1 | Cosoleacaque | 1/1 |
| 48. 33/1 Tornado/A-7573 | 1 | Cosoleacaque | 1/1 |
| 53. 1/10 A-7573/criollo | 1 | Cosoleacaque | 1/1 |
| 57. 1/55 A-7573/N.I | 1 | Cosoleacaque | 1/1 |
| 58. 6/10 VS-536/criollo | 1 | Cosoleacaque | 0/1 |
| A-7573 | 117 | Cosoleacaque; Paso de Ovejas; Tlalixcoyan | 44/117 |
| CP | 1 | Tlalixcoyan | 1/1 |
| CP-560A | 3 | Cosoleacaque; Tlalixcoyan | 2/3 |
| CP-562 | 1 | Tlalixcoyan | 0/1 |
| CP-562A | 3 | Cosoleacaque; Tlalixcoyan | 2/3 |
| Criollo | 13 | Cosoleacaque | 9/13 |
| H-520 | 4 | Cosoleacaque; Medellin; Tlalixcoyan | 2/4 |
| HQ1 | 3 | Cosoleacaque; Tlalixcoyan | 3/3 |
| HQ2 | 4 | Cosoleacaque; Tlalixcoyan | 2/4 |
| HQ3 | 1 | Tlalixcoyan | 0/1 |
| HQ4 | 3 | Cosoleacaque; Tlalixcoyan | 3/3 |
| Nutria | 2 | Cosoleacaque; Tlalixcoyan | 2/2 |
| T-501 | 1 | Tlalixcoyan | 0/1 |
| Tornado | 8 | Cosoleacaque | 7/8 |
| V-536C | 1 | Tlalixcoyan | 0/1 |
| V-537C | 1 | Tlalixcoyan | 0/1 |
| V-556AC | 1 | Tlalixcoyan | 1/1 |
| VAN-543 | 2 | Cosoleacaque; Tlalixcoyan | 2/2 |
| VS-536 | 11 | Cosoleacaque; Tlalixcoyan | 9/11 |
| VS-543R | 2 | Cosoleacaque; Tlalixcoyan | 2/2 |
| ND | 38 | Cosoleacaque; Medellín; Paso de Ovejas; Tlalixcoyan | 11/38 |
We cannot discard that the presence of seasonal vectors pose an increase pressure of viruses to the host (maize) plants, in the different regions and seasons of the year. For instance, among the different vectors for the viruses detected in this report, like aphids, leafhoppers, nematodes and even plasmodiophora, the first group increases in populations during fall and winter when most of the samples were collected. Undoubtedly, more studies about virus identification, occurrence and distribution must be made in Mexican maize fields.
Conclusions
We found simultaneous infections with more than two viruses in maize plants with or without CSS in Veracruz, Mexico. The results obtained in this study, agree with reports about the high occurrence and extensive dissemination of CSS, revealing how this pathogen, can associate with maize viruses and increase corn diseases severity in Mexican fields.
Although the survey was made during 2006-2007, this study becomes important because is the first report of novel associations between CSS and viruses SCMV, MCMV, MSpV and MWLMV in Mexico.











 texto en
texto en 


