Introduction
Biological control can be defined as a reduction in the amount of inoculum or disease produced by the activity of a pathogen, based on the use of natural enemies or use of compounds derived from its metabolism (Soria et al., 2012; Harding & Raizada, 2015; Sundin et al., 2016). It offers an alternative to chemical products, helping to minimize the negative consequences for human health and environment (O’Brien, 2017). Biological control agents are frequently tested, developed and used as an effort to control several soil borne plant pathogens (Yobo et al., 2010). Endophytic bacteria can be defined as those that can be isolated from healthy plant tissues and superficially disinfected without causing any damage to the host plant (Compant et al., 2005; Miliute et al., 2015); they are capable of penetrating and disseminating systemically in the host plant, actively colonizing the conductive vessels, and occasionally the intracellular spaces. This colonization presents an ecological niche, similar to that occupied by plant pathogens, and these endophytic bacteria can act as biological control agents against pathogens (Dai et al., 2016). Biological control of plant pathogens is the result of the production of antifungal or antibacterial agents, siderophores, the competition of nutrients (Sturz et al., 2000) and systemic acquired resistance induction of the host or immunity (González et al., 2015), increasing the availability of minerals (Sessitsch et al., 2002). Endophytic bacteria are used for their capacity to produce antibiotics (Maksimov et al., 2011), and enzymes such as chitinases, glucanases, proteases and lipases, which cause cell lysis (Neeraja et al., 2010). They are also known for their ability to control plant pathogens such as Fusarium oxysporum f. sp. vasinfectum causing wilt in cotton (Chen et al., 1995), fungi such as Rhizoctonia solani and F. oxysporum in potato cultivation (Castro et al., 2017), Oomycete Phytophthora cinnamomi causing the root rot of avocado (Pérez et al., 2014); another study showed that strains of endophytic bacteria isolated from vanilla plants inhibited the growth of F. oxysporum f. sp. vanillae through antibiosis and competition for space and nutrients (Jiménez et al., 2015). They also established a symbiotic association, which produces great benefits for plants, promoting plant growth (Pérez et al., 2013). The effect of endophytes on plant growth and development is due to direct or indirect mechanisms of action. Among the direct mechanisms is the production of hormones such as auxins, gibberellins, cytokinins and ethylene. The production and mobilization of organic acids, fixation of nitrogen, solubilization of phosphate and other nutrients (Sevilla et al., 2001, Hurek et al., 2002, Iniguez et al., 2004, Khalifa et al., 2015). The elucidation of the mechanisms that promote the growth of plants helps to favor the species and the conditions that lead to greater benefits for the plants. Volatile substances such as 2-3 butanediol and acetoin produced by bacteria appear to be a mechanism responsible for promoting the growth of plants (Ryu et al., 2003). The production of phytohormones is considered one of the most important mechanisms to promote plant growth, these organic compounds regulate growth and development in plants, and in low concentrations influence biochemical, physiological and morphological processes. AIA (3-indole acetic acid) is the most common phytohormone, best characterized and physiologically most active auxin in plants (Soler et al., 2012), AIA is responsible for division, expansion and differentiation of cells and plant tissues and stimulates roots elongation (Martínez et al., 2010, Rojas et al., 2016). The objective of the present work was to evaluate the effect of the application of two consortia of endophytic bacteria in a Pinto Saltillo´s bean cultivar to control R. solani and F. oxysporum fungi and to determine their effect on growth and development under greenhouse conditions.
Material and Methods
Isolation and identification of soil borne plant pathogens
The plant pathogens were obtained from bean plants (Phaseolus vulgaris) Pinto Saltillo cultivar with wilt symptoms, they were collected in experimental fields of Universidad Autónoma Agraria Antonio Narro, Saltillo, Coahuila, which were cultivated under rainfed farming conditions, fragments of roots and stems were placed in PDA culture medium and incubated at 26 ± 2 ° C for seven days. The fungus F. oxysporum was purified by monosporic cultures and characterized phenotypically according to the morphological characteristics described by Leslie & Summerell (2006), while R. solani was purified by hyphae tip and characterized according to the criteria of Sneh et al. (1991). The isolates were preserved at 4 °C.
Extraction of DNA from fungi
Starting from axenic strains, each petri dish with fungal mycelium was scraped with a spatula trying to recover as much as possible, the result of each petri dish was deposited in an ependorff tube, giving a total of four ependorf tubes for each species. 500μL of extraction buffer containing 10m M EDTA, 10 mM Tris HCl pH 8.0, SDS 0.5 % was added to the recovered material and vortexed for 15 min. Subsequently, 500 μL of the lower phase of the Phenol-Chloroform solution (1:1) was added and a vortexed for 15 min, centrifuging at 12,000 rpm for 10 min. The aqueous phase was recovered in a new tube and 1.0 μL of RNAse (20 mg/mL) was added, incubating for 30 min at 37 °C in the thermomix. Then, 0.33 volumes of Isopropanol were added at -20 °C, mixing gently and incubating for 30 min. To form the pellet, centrifugation was performed at 12,000 rpm for 10 min. The supernatant was then decanted and the pellet was washed with 500 μL of 70 % ethanol, then centrifuged at 12,000 rpm for 5 min. It was decanted and the pellet allowed to dry under a laminar flow hood for approximately 15 min. The DNA was resuspended in 25 μL of TE. The integrity and quality of the extracted DNA was verified in a 1 % agarose gel, under horizontal electrophoresis applying a voltage of 100 volts for 40 min (Nicholson et al., 2001).
Amplification by polymerase chain reaction (PCR)
For the amplification of the ITS1 and ITS4 region (Internal Transcribed Spacer), an amplification mixture was made in a final volume of 20 μL, composed of 13.58 MQ water, 2.0 μL of MgCl buffer (10X), 0.32 μL of MgCl2 (25mM), 0.4 μL of dNTP›s (10mM), 0.5 μL of each ITS1 primer (5›TCC GTA GGT GAA CCT GCG G3›) and ITS4 (5›TCC TCC GCT TAT TGA TAT GC3›) (10μM), 0.5 μL of DMSO, 0.2 μL of DNA Taq-polymerase 1U and 1 μL of DNA (40 ng/μL). The amplification reactions were carried out using a thermal cycler (Bio-Rad T100 ™ Thermal Cycler) under the following program: 1 cycle of 94 °C 3 min, followed by 35 cycles at 94 °C 45s, 53 °C for 45s and 72 °C 1 min; finishing with a polymerization cycle of 72 °C for 7 min. The amplified bands were observed in a 1.0 % agarose gel at 90V for 60 min.
Sequencing of PCR products
The PCR product was increased and purified by GeneAll®ExpinTM PCR SV kit, which were sequenced by the Macrogen laboratory. The sequences obtained were analyzed in the GenBank database of the National Center for Biotechnology Information (NCBI), using the BLAST program (Basic Local Aligment Search Tool) for highly similar sequences. Each sequence obtained was depured in its initial and final part to increase the sensitivity of the analysis.
Biocontrol of R. solani and F. oxysporum with formulations of endophytic bacteria and their effect on growth and development of beans under greenhouse conditions
Pinto Saltillo cultivar bean seeds free from any chemical product provided by the Germplasm Bank of the Universidad Autónoma Agraria Antonio Narro were used, two seeds of the study crop were sown in 5 Kg pots with pasteurized soil obtained of bean fields of this University. At the time of planting, the soil borne plant pathogens were inoculated; for the case of F. oxysporum a suspension of 1x106 conidia/mL was used, for R. solani a suspension at 1x106 propagules/mL, prepared by macerating 4 Petri dishes with PDA and growth of R. solani of 14 days of age in 1 L of sterile distilled water. Each species of fungus was applied with a hypodermic syringe, depositing a volume of 25 ml of the suspension of each fungus on the bean seeds. The endophytic bacteria were isolated and formulated by Green Corp Biorganiks of Mexico S.A. of C.V. Company. The formulations contain a mixture of Bacillus species: the difference between the formulations consists of different conditioners in each of these which influences the stability and biological viability of the prototypes. Three applications were made: the first at the time of sowing, the second after the emergence of the plants when they reached about 15cm in height and the third was at an interval of 15 days after the second. The applications were made with a manual sprinkler. The treatments studied were: (T1) pathogen + bioformulated 1 - 1x106, (T2) pathogen + bioformulated 2 - 1x107, (T3) bioformulated 1 - 1x106, (T4) bioformulated 2- 1x107, (T5) pathogen, (T6) control, it should be mentioned that the experiments were carried out separately, one for R. solani and another for F. oxysporum. The experiment was established in a random block design under greenhouse conditions with seven repetitions per treatment, with 42 experimental units, two plants per experimental unit, giving a total of 84 plants for each experiment. We chose to use a randomized block design because the conditions inside the greenhouse were not homogeneous since the site is not 100 % conditioned.
The disease incidence was determined and expressed as percent of diseased plants. The severity was assessed with a six-class scale; where: 0 - Plants with healthy stems and roots, 1 - Plants with minimal damage in stems and roots (less than 10 %), 2 - Plants with slight damage in stems and root (25 %), 3 - Plants with medium damage in stems and root (50 %), 4 - Plants with severe damage in stems and root (75 %) and 5 - Plants with dead stems (100 %) (Castro et al., 2017). The incidence and severity variables had an arcsine transformation of the square root of the observed value.
The effect of the bioformulates in the promotion of plant growth and development, some parameters were measured, such as; plant height expressed in cm, stem diameter expressed in mm, in addition the relative content of chlorophyll was determined with a SPAD 502 Plus.
Results and Discussion
Isolation and identification of soil borne plant pathogens
The morphological characteristics of F. oxysporum observed in potato dextrose agar culture medium were cottony mycelium and variable color according to age, from a pinkish to a pale violet at two weeks. The microconidia were observed without septa, oval, elliptical to reniform, formed in abundance in monofialides in bottle shaped short called “false head. The chlamydospores of intercalary or terminal position, individually or in pairs, with a smooth circular double-wall appearance. The macroconidia observed were fusoid and allantois with three to five septa and the hook-shaped. In a Carnation-agar medium (CLA) a hyaline mycelium was observed, growing very close to the surface of the medium, after 10 days the sporodochia were formed in the carnation leaf. This characteristic corresponds to that described by Leslie & Summerell, (2006). The characteristics of R. solani were colorless mycelium when young, but turned yellowish to light brown at two weeks of age, with elongated cells and branches that grew at approximately right angles to the main hypha, were slightly constricted at the junction, and they had a transverse wall near the junction. This characteristic was considered basic for the identification of Rhizoctonia solani (Sneh et al., 1991). The sequences of the fungi compared in BLAST identified Fusarium oxysporum strain Af / 8/1 with access code JN624887.1 with 99 % identity and Rhizoctonia solani with access code JX050236.1 with 99 % identity, this confirms the identification morphological of pathogens.
Biocontrol of R. solani and F. oxysporum with formulations of endophytic bacteria and their effect on the growth and development of beans under greenhouse conditions
Incidence and severity of the disease
There were statistically significant differences between the treatments, for the variables of incidence and severity of the disease by R. solani and F. oxysporum evaluated in bean plants (Table 1).
Table 1 Mean square of the error between treatments and value of P, for incidence and severity of R. solani and F. oxysporum in the bean crop.
| Variable | CME* | P>F |
|---|---|---|
| Incidencia de F. oxysporum | 0.0 | <.0001 |
| Severidad de F. oxysporum | 278.57 | <.0001 |
| Incidencia de R. solani | 0.0 | <.0001 |
| Severidad de R. solani | 192.85 | <.0001 |
*Mean square of the error.
Although the analysis showed significant differences, the microbial formulations had no effect on the incidence of fungi F. oxysporum and R. solani, since they were developed in all the treatments where they were inoculated, however the data indicate that they partially reduced the severity of these plant pathogens, the range was from 0.0 % to 62.14 % for F. oxysporum and from 0.0 % to 83.57 % respectively. Bioformulate 1 was more effective for F. oxysporum while bioformulate 2 was for R. solani (Table 2). Another study previously conducted by Ben et al. (2016) reported that isolates of Alcaligenes faecalis S18 and Bacillus cereus S4 also significantly reduced Fusarium wilt in tomato caused by F. oxysporum f. sp. lycopersici.
Table 2 Effect of two formulations of endophytic bacteria on the incidence and severity of Fusarium oxysporum and Rhizoctonia solani in bean plants.
| Treatment | Incidence (%) | Severity (%) |
|---|---|---|
| 1. F. oxysporum + bioformulate 1 - 1x106 | 90 a | 45.00 a |
| 2. F. oxysporum + bioformulate 2 - 1x107 | 90 a | 62.14 a |
| 3. Bioformulate 1 - 1x106 | 0 b | 0 b |
| 4. Bioformulate 2 - 1x107 | 0 b | 0 b |
| 5. Fusarium oxysporum | 90 a | 62.14 a |
| 6. Control | 0 b | 0 b |
| 1. R. solani + bioformulate 1 - 1x106 | 90 a | 77.14 a |
| 2. R. solani + bioformulate 2 - 1x107 | 90 a | 70.71 a |
| 3. Bioformulate 1 - 1x106 | 0 b | 0 b |
| 4. Bioformulate 2 - 1x107 | 0 b | 0 b |
| 5. Rhizoctonia solani | 90 a | 83.57 a |
| 6.Control | 0 b | 0 b |
Means with arcsine transformation of the square root of the observed value, columns followed by the same letter are not statistically different according to Tukey’s mean separation test at 0.05 % significance.
Plant height and stem diameter
On the other hand, the analysis of the effect of bioproducts on bean plants inoculated with R. solani or F. oxysporum revealed that the height and diameter of the stem increased in the treatments that received applications of the bacterial formulations. Formulated 1 and 2 in the absence of soil borne plant pathogen fungi stood out for their stimulating effect on the growth of the plants under study. This growth stimulating effect was also seen in the plants treated with the formulations and inoculated with the pathogens at the same time (Figure 1). The plants inoculated only with the plant pathogens F. oxysporum or R. solani showed a more limited development. It is important to mention that the plants grew under no chemical treatment, therefore they did not receive fertilization by any chemical source. Other recent studies, conducted by Castro et al. (2017) show that endophytic Bacillus bacteria applied as a consortium are able to promote plant growth in potato cultivation, obtaining favorable results in height and diameter of stem as well as fresh weight of biomass and tuber. According to the information reported by Santoyo et al. (2015), various bacterial endophytes can promote the growth of plants as a consequence of direct or indirect mechanisms. The direct promotion of plant growth occurs when a bacterium facilitates the acquisition of essential nutrients or regulate the level of hormones within the plant. The acquisition of nutrients facilitated by endophytes generally includes nitrogen, phosphorus and iron. The regulation of hormone levels may imply that one or more of the phytohormones are synthesized as auxins, cytokinins and gibberellins. Indirect promotion of plant growth occurs when bacteria decrease damage to plants after infection with a phytopathogen that includes some soil fungi and bacteria. Other studies by Ryu et al. (2003) demonstrated that strains of endophytic bacteria such as Bacillus subtilis and B. amyloliquefaciens had the ability to promote significant growth in Arabidopsis.
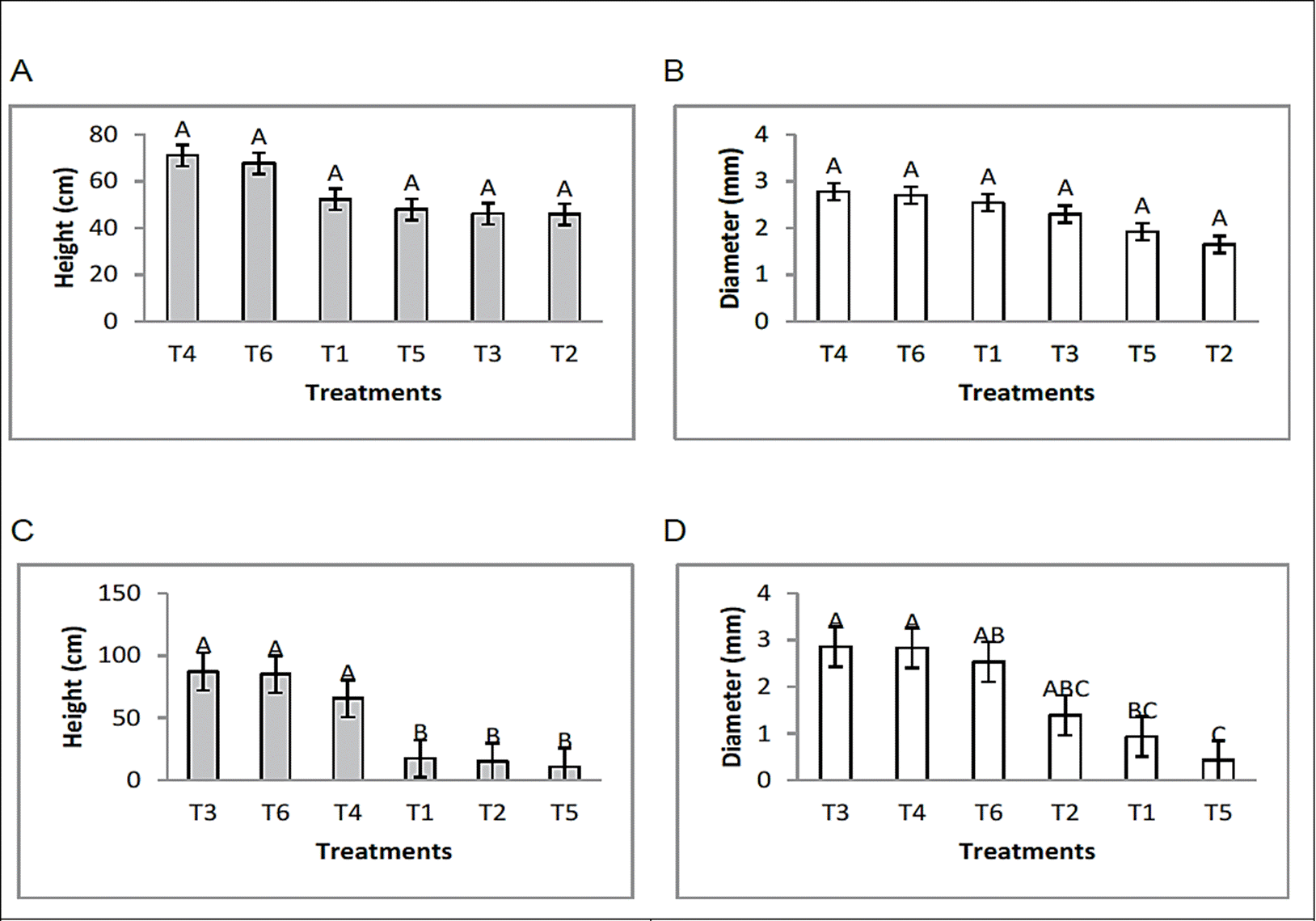
Figure 1 Effect of endophytic bacteria on plant height and stem diameter in bean crop under greenhouse conditions. [(F. oxysporum; (A) height, (B) diameter) (R. solani; (C) height, (D) diameter)]. Means with the same letter are not significantly different according to the Tukey tested at p=0.05. Error bars are standard error of the mean.
Chlorophyll content
Chlorophyll levels in bean plants increased remarkably, the inductor effect that the formulated had on the relative amount of chlorophyll was demonstrated, formulated 1 and 2 in the presence and absence of plant pathogens showed levels higher than those found in controls (Figure 2). In this section, a general discussion is made as there are no reports where they relate the chlorophyll content to the attack of plant pathogens.
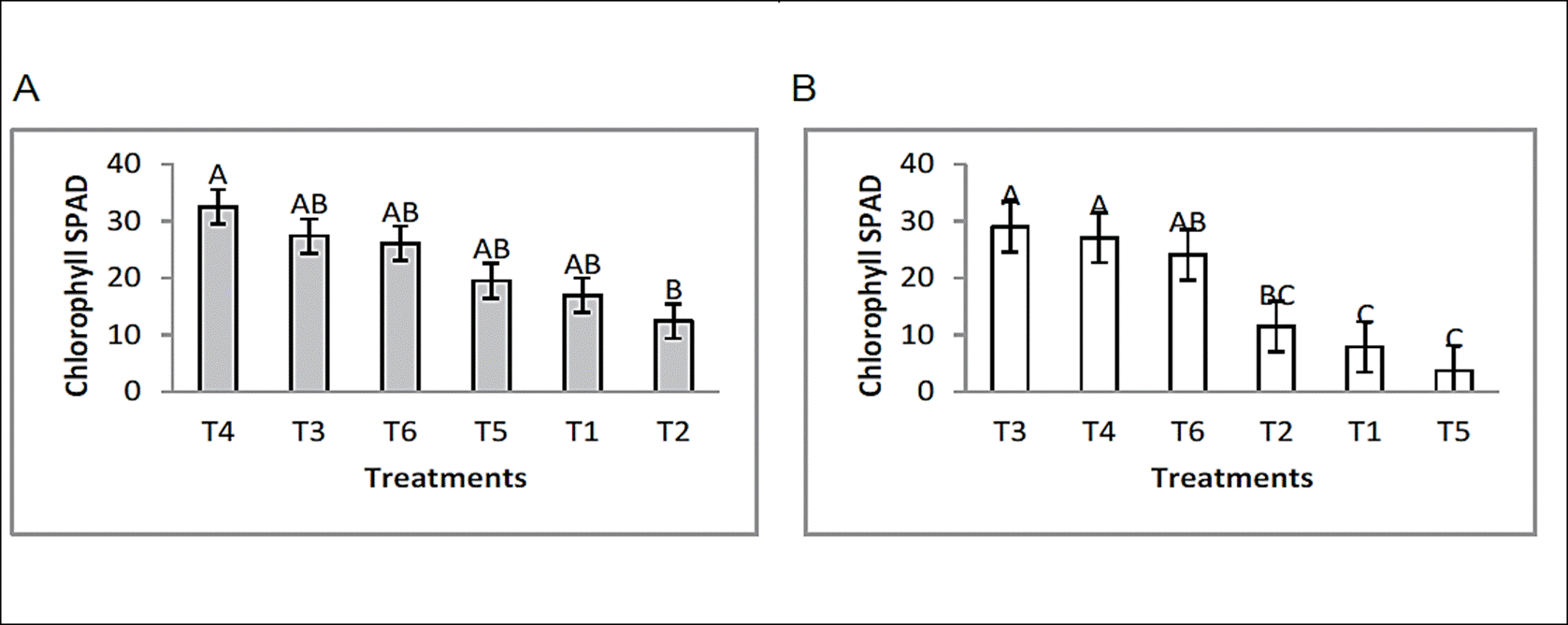
Figure 2 Effect of endophytic bacteria application on chlorophyll content, under greenhouse conditions. [F. oxysporum; (A) and R. solani; (B)]. Means with the same letter are not significantly different according to the Tukey tested at p=0.05. Error bars are standard error of the mean.
Therefore, Turner & Jund, (1991), with a chlorophyll detector model SPAD-502, demonstrated that the “SPAD unit” is a value proportional to the nitrogen content, so in this study it is intended to relate the chlorophyll content in relation to plant growth, where the endophytic bacteria used to improve chlorophyll levels is reflected in plant growth. The results showed that F. oxysporum and R. solani significantly affected the production of chlorophyll, compared with the treatments where the bioformulates were applied which induced the highest rates and were not affected. Chlorophyll content and nitrogen absorption have been correlated with SPAD units in various environmental conditions such as light intensity, temperature, relative humidity, pests, population density, nitrogen source, etc. (Hiderman et al., 1992).
Conclusion
The formulations based on endophytic bacterial strains used in this study showed favorable effectiveness as biocontrol agents against the fungi Fusarium oxysporum and Rhizoctonia solani in disease severity, in addition the formulated ones significantly increased the growth of bean crop in comparison with control.











 texto en
texto en 


