Introduction
The Amazonia and Atlantic Forest are the two major tropical rainforests of South America (Hueck 1972). These two large forests are currently separated by the dry forests of the Caatinga, the Chaco shrublands and the savanic Cerrado (Ab’Saber 1977; Solari et al. 2012). Paleoclimatic, biogeographic, and niche modeling studies have suggested intermittent past connections between Amazonia and Atlantic Forest, during the last interglacial period (Wang et al. 2004; Sobral-Souza et al. 2015; Ledo and Colli 2017).
Traditionally, the disjunct distribution pattern of rainforest-adapted mammals has been considered as evidence that the Amazonia and Atlantic Forest were connected (Coimbra-Filho and Câmara 1996; de Vivo 1997). Notable examples include medium and large arboreal species such as the red-handed howler (Alouatta belzebul), the kinkajou (Potos flavus), and the silky anteater (Cyclopes didactylus). The existence of similar patterns for small mammals, such as bats, has been little investigated (but see Costa 2003 and Rocha et al. 2015). To explain these biogeographic patterns, hypotheses often invoke the fragmentation of forests that occurred during the Pleistocene (Vanzolini and Williams 1970; Martins 1971; Haffer 1997). One way to test this hypothesis is to obtain paleoclimatic data from this epoch (Vanzolini and Williams 1970), and to examine the divergence times and amount of genetic divergence among the involved species (Moritz 2000).
MacConnell’s Bat, Mesophylla macconnelli Thomas, 1901, is one of the smallest species of frugivorous bat in the world, weighing 6 to 8 g (Solari et al. 2019). This tent-roosting bat has been recorded from the rainforests of Nicaragua, to the Amazon basin in South America, reaching northern Bolivia and western Brazil (Arroyo-Cabrales 2008). However, recent studies extended its range to the Atlantic Forest and to the Cerrado of, respectively, eastern and central Brazil (Zortéa and Tomaz 2006; Gregorin et al. 2014). With the new records, the disjunct distribution pattern of M. macconnelli is strikingly similar to what has been observed for other forest-dependent mammals that occur in both the Amazonia and Atlantic Forest. A recent study identified 127 species of mammals that occur in both ecosystems, suggesting them as good candidates for phylogeographic studies that investigate this putative vicariant pattern, but M. macconnelli was not mentioned by the authors (Machado et al. 2021).
Using paleoclimatic data and ecological niche models, we estimate the past potential distribution of M. macconnelli during the last 130,000 years. Our objective is to assess the potential for past connections between the Amazonia and Atlantic Forest that may explain the apparently disjunct distribution pattern of the species.
Materials and methods
Mesophylla macconnelli occurrence data. Occurrence records for M. macconnelli were obtained from museum specimens held in the Instituto de Desenvolvimento Sustentável Mamirauá (IDSM), Natural History Museum, London (BMNH), Universidade Federal de Lavras (CMUFLA), Museu de Zoologia da Universidade de São Paulo (MZUSP), Universidade Federal de Mato Grosso (UFMT), Universidade Federal de Minas Gerais (UFMG), and National Museum of Natural History, Smithsonian Institution (USNM).
We also incorporated secondary records in online databases such as Global Biodiversity Information Facility - GBIF (www.gbif.org), SpeciesLink (http://splink.cria.org.br/) and Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio (https://biodiversidade.icmbio.gov.br/). Additionally, existing records in the scientific literature were included (see Supplementary material 1).
Our database went through a process of cleaning and removing records with missing or repeated geographic coordinates and records outside the known geographic distribution of the species. After this procedure, a single occurrence record was randomly selected within an area equivalent to two cells of resolution of the environmental layers (each cell = 9.24 x 9.24 km; Velazco et al. 2019). This was to prevent sampling bias from propagating biased ecological niche models.
Environmental Data. The Neotropical region was determined as our study area to calibrate our model (Olson et al. 2001), considering the wide distribution range of M. macconnelli in Central and South America, and potential dispersion ability. Ecological niche models (ENM) for current conditions were adjusted based on 19 bioclimatic variables related to temperature and precipitation (Hijmans et al. 2005). Detailed information about each variable is available in Supplementary material 2, Table S1. Paleoclimatic conditions for the Last Interglacial (LIG; 120,000 to 140,000 years ago) and Last Glacial Maximum (LGM; ~21,000 years ago) were obtained from the PaleoClim.org database (Brown et al. 2018). All variables were obtained at the resolution of 5 arcminutes and were cropped to the same extent of our study area. To avoid overfitting and to assess correlation among biotic variables a Pearson’s correlation test was applied to the bioclimatic variables. This test was performed using the “raster.cor.matrix” function of the ENMTools package in R (Warren et al. 2021). From each pair of strongly correlated variables, i. e., r ≥ |0.7|, we kept the one with the highest biological meaning for the species (Da Silva et al. 2020). After this procedure, five variables remained: Mean Diurnal Range (bio2), Temperature Seasonality (bio4), Max Temperature of Warmest Month (bio5), Annual Precipitation (bio12), and Precipitation Seasonality (bio15).
Modelling procedures. We used four algorithms to construct ENM: Bayesian Gaussian - GAU (Golding 2014), Maximum Entropy - MXD (Phillips et al. 2017), Random Forest - RDF (Liaw and Wiener 2001), Support Vector Machine - SVM (Karatzoglou et al. 2004). The same number of pseudoabsences were generated to fit the models (Barbet-Massin et al. 2012). An environmental restriction method was used to allocate the pseudoabsences in climatically different regions of the environmental space in which the species occurs (Engler et al. 2004). We used the checkboard method to calibrate and evaluate models. This method consists of dividing the geographic space into blocks, splitting the occurrences into two groups, one for model adjustment and another for model evaluation (Roberts et al. 2017).
Model performance was evaluated using two metrics: Area Under Curve (AUC) Receiver Operating Characteristic (ROC; Phillips et al. 2006), and True Skill Statistic (TSS = sum of sensibility and specificity - 1; (Allouche et al. 2006). AUC ranges from 0 to 1, where values closer to 1 indicate a good distinction between presence and pseudoabsence records. Whereas, values below 0.5 indicate that the model did not perform better than expected by chance (Fielding and Bell 1997). TSS ranges from -1 to +1, values above 0.7 indicate models with statistically reliable performance (Allouche et al. 2006; Zhang et al. 2015). To reduce the uncertainty in the prediction generated by the use of distinct algorithms, we build ensemble models (Araújo and New 2007). The ensemble model was calculated selecting models with TSS value greater than the average TSS value of all algorithms and then calculated the mean suitability model between all algorithms that met this condition. Finally, we projected the current climatic suitability conditions for M. macconnelli under past climatic conditions (LIG, ~ 120,000 years ago; LGM, ~21,000 years ago) for each algorithm and then created an ensemble model for each period. For LGM we also used different climatic conditions estimated by 3 distinct Atmosphere-Ocean General Circulation Models (AOGCMs): CCSM4, MIROC-ESM e MPI-ESM-P (Hijmans et al. 2005). Specifically, for LGM models a final model was created by calculating the average suitability values obtained through the three AOGCM’s ensemble models. Finally, we use the threshold that maximizes the sum of sensitivity and specificity (Liu et al. 2005), to turn continuous suitability values into binary presence-absence models.
To test the hypothesis of connection between the distribution of M. macconnelli through Amazon and Atlantic Forest, we overlapped the ensemble models of the three time periods. In this way we could identify areas of climate stability, or areas of connection and reconnection that may have been lost in the species current distribution.
Results
Current records. We found 681 records of Mesophylla macconnelli in Central and South America and after the filtering process, 260 unique records were used in modelling procedures. Most of the records (565, 83.21 %) are located east of the Andes, but some (114, 16.79 %) occur west of the Cordillera (Figure 1). The majority of the records (627, or 92.7 %) are in Tropical & Subtropical Moist Broadleaf Forests, of which 625 are in the Amazon rainforest and two from the Atlantic Forest. Some records are from the savanna formations of the Cerrado (33, or 4.85 %) and Tropical and Subtropical Dry Broadleaf Forests (21, or 3.08 %). Of all the occurrence points, 85.5 %, or 590, are from altitudes below 900 m above sea level. The remaining 91 localities are from above 900 m, with a maximum altitude of 2,355 m from the cloud forests of Cuzco, Perú.
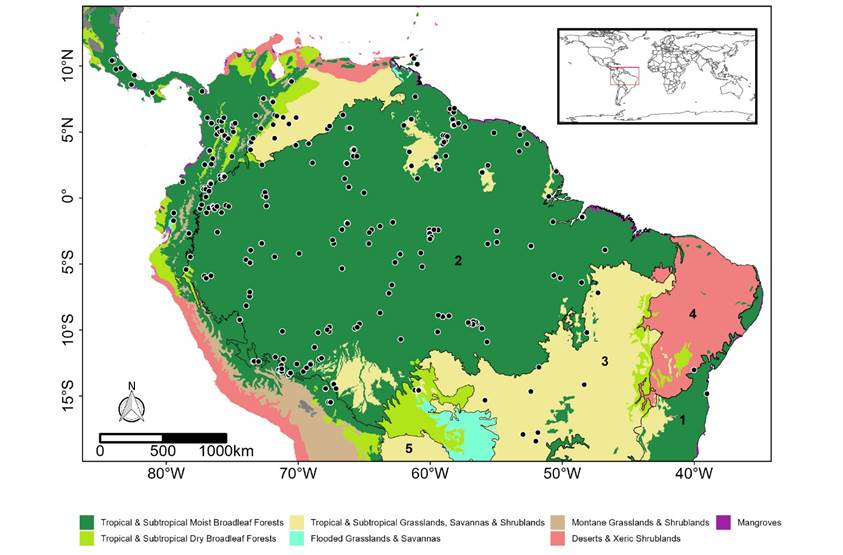
Figure 1 Occurrence records of MacConnell’s Bat (Mesophylla macconnelli) in the American continent. Colors on map indicate the biomes classified according to Olson (2001). Numbers show the ecoregions of the Neotropical Realm following Olson (2001) (1 - Atlantic Forest, 2 - Amazon Forest, 3- Cerrado, 4 - Caatinga, 5 - Chaco).
Geographic distribution models. The evaluation values for the ensemble models, AUC = 0.998 SD=0.028 and TSS = 0.980 SD = 0.027, indicated good performance of the models - for more information about other evaluation metrics and evaluation for each algorithm see Supplementary Material 3. The temperature seasonality (45.3 %) and annual precipitation (36.4 %) provided the highest relative contributions to model M. macconnelli distribution. The current distribution model shows a wide area with high suitability values in the Amazon and in the central and northern areas of the Atlantic Forest, with suitable areas also in the Cerrado biome (Figure 2).
During all three projected periods (i. e., LIG, LGM, and Current), the Chocó region, Panama, western Amazonia, and central/northern Atlantic Forest were estimated as highly suitable areas for M. macconnelli (Figure 2). The projected distribution for the LIG shows that areas in the southern part of the Amazon, in the Cerrado, and in the Atlantic Forest had suitable climatic conditions, showing a possible connection between the southwestern Atlantic Forest and southern Cerrado (Figure 2). During the LGM we infer a great expansion of appropriate areas towards northern and eastern Amazonia (Figure 3). This occurs concomitantly with a retraction of the southern distribution of the species. This pattern suggests the loss of the connection between the appropriate areas of the Cerrado and Atlantic Forest.
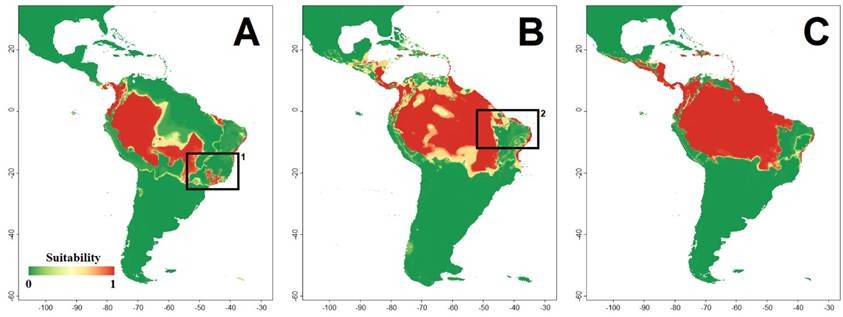
Figure 2 Ecological niche models (ENM) projections of MacConnell’s Bat (Mesophylla macconnelli) during the (A) Last Interglacial (LIG) - ~120 000 years ago, (B) Last Glacial Maximum (LGM) - ~21 000 years ago, and (C) current distribution. Ensemble models were adjusted based on four algorithms (Bayesian Gaussian - GAU (Golding 2014), Maximum Entropy - MXD (Phillips et al. 2017), Random Forest - RDF (Liaw and Wiener 2001), Support Vector Machine - SVM (Karatzoglou et al. 2004). Black empty squares indicate possible connections between Atlantic Forest and Amazonia on LIG (1) and LGM (2). The color scale indicates suitability values for M. macconnelli occurrence.
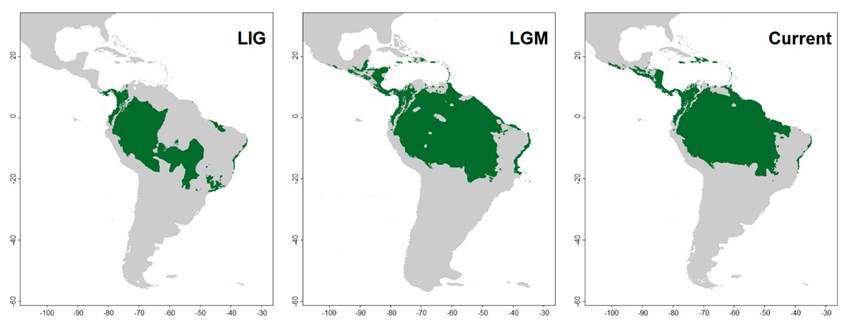
Figure 3 Distributional binary maps of MacConnell’s Bat (Mesophylla macconnelli) during the transition from the Last Interglacial period to the Last Glacial Maximum (LIG - LGM) and from the Last Glacial Maximum to the current period (LGM - Current). Ensemble models were adjusted based on four algorithms (Bayesian Gaussian - GAU (Golding 2014), Maximum Entropy - MXD (Phillips et al. 2017), Random Forest - RDF (Liaw and Wiener 2001), Support Vector Machine - SVM (Karatzoglou et al. 2004). Green areas indicate predicted species occurrence. Species absence throughout the study area is represented in gray.
Discussion
The current distribution of M. macconnelli seems to be associated with humid forested areas. Even in localities within the Cerrado biome, such as Serra do Roncador (Mato Grosso state, Brazil), and Serranópolis (Goiás state), it has been captured in forest enclaves and riparian areas (Pine et al. 1970; Handley 1976; Zortéa and Tomaz 2006). In fact, these transitional areas and forest enclaves in the Cerrado are known to harbor some typical Amazonian mammalian taxa such as Ateles marginatus, Callicebus vieirai, Chiroderma trinitatum, Didelphis marsupialis, Gracilinanus peruanus, Marmosops noctivagus, and Saguinus niger (Lacher and Alho 2001; Antunes et al. 2021; Garbino et al. 2015, 2020; Lima-Silva et al. 2022; Semedo et al. 2022). Besides forests, temperature seasonality, calculated as the standard deviation of monthly temperature averages, seems to be a limiting factor for the species. This is evident, as M. macconnelli occurs in tropical areas between 10° N and -18° S, where there are no abrupt temperature oscillations (Figure 1).
The species is absent in the cooler areas of the Atlantic Forest of southern and southeastern Brazil, which have more seasonal climates than the central Atlantic Forest, where the species occurs. This hypothesis seems more plausible than assuming that the drier formations of the Cerrado acted as a barrier, especially because the Amazon and Atlantic Forest were recently connected by riparian corridors in the region of Goiás, Brazil, where M. macconnelli has been recorded (Ab’Saber and Costa-Junior 1950). The dependence on leaves modified into tents to use as daytime roosts (Rodriguez-Herrera et al. 2007; Garbino and Tavares 2018) may help explain why the distribution of M. macconnelli is intimately associated with forests.
According to our model, in the last interglacial period the Andes cordillera acted as a barrier for the species, with suitable areas on the east and west slopes but not in the Andean highlands and plateaus (Figure 2). However, the model suggests that the current period allows for more permeability between the transandean and cisandean populations, which may explain the low genetic structure found in the species (Tavares et al. 2022). The model considering the current distribution also indicates that the populations from the Atlantic Forest (eastern Brazil) are disconnected from the Amazonia/Cerrado populations (Figures 2 and 3). Future surveys in poorly sampled areas where the presence of M. macconnelli is not known, may show if the absence of the species in these areas is due to sampling deficiencies.
Some historical biogeographic patterns observed in other vertebrates are suggested in our projected distribution of M. macconnelli. The western Amazonia, an area considered an important center of diversity for vertebrates (Hoorn et al. 2010; Oliveira et al. 2017), is recovered as suitable in all three periods (Figure 3). The area where the species occurs in the Atlantic Forest, with climatic stability during all three periods (Figures 2, 3), is known as “Bahia Refuge” and has been identified as a stable climatic area based on other species of vertebrates (Carnaval and Moritz 2008).
The modelled distribution of M. macconnelli suggests at least two historical periods of connection between the Atlantic Forest and the Amazonia (Figure 2). In the Last Interglacial, where the climate was more humid, our models recovered suitable areas to the southwest (Figure 2). In the Last Glacial Maximum, when the climate was cooler and drier, our model recovered a northeastern connection of M. macconnelli’s range (Figure 2A, B). These two connections may have formed repeatedly over the Pleistocene climatic oscillations, with the southwest connection between Amazon and Atlantic forests the more ancient and frequent, and the northeast the more recent one (Ledo and Colli 2017). This scenario may have led to the apparent disjunct distribution of M. macconnelli. In another forest-dwelling frugivore bat, Carollia perspicillata, there is genetic evidence of geographically restricted intraspecific lineages that reflect Pleistocene glacial cycles (Pavan et al. 2011).
Future phylogeographic studies, including genetic samples from the Cerrado of central Brazil, the Atlantic Forest of eastern Brazil, and from eastern and southern Amazonia, will allow verification of the pattern suggested here. We also suggest that niche modelling based on past climates may open new venues of investigation on the biogeographic patterns of the Neotropical fauna.











 nueva página del texto (beta)
nueva página del texto (beta)


