Introduction
Strandings represent a unique opportunity to collect information about elusive cetaceans (Canto et al. 1991; Leeney et al. 2008; Pikesley et al. 2011). Although these events are frequent across different coastal areas of the planet (Evans et al. 2005; Berta et al. 2015; Moore et al. 2018), the frequency of such stranding events differs among species (Leeney et al. 2008). In the case of species of the family Ziphiidae, records are scarce, and many of them are based on the stranding of specimens in an advanced state of decay or isolated bone remains (Litcher 1986; Dalebout et al. 1988). Therefore, only a few high-quality photographic records of ziphiids exist, given that instances of complete individuals in good condition are exceptional (Cox et al. 2006). Traditionally, identifying specimens of Mesoplodon at the species level has been a complex task (Dalebout et al. 2002, 2004; Van Helden et al. 2002; Cappozzo et al. 2005). For example, relying on body patterns of coloration (a trait usually used for species identification) can sometimes result in wrong taxonomic identifications (Helden et al. 2002; Dalebout et al. 2014) due to the insufficient knowledge about intra and interspecific color variation as well as a poor understanding of how coloration varies after death.
The ziphiid genus Mesoplodon is the most diverse, accounting for 15 of the 22 species of the family (Cappozzo et al. 2005; McLeod et al. 2006; Pitman 2009; McLeod 2009, 2017; Yamada et al. 2019). Ten species of Mesoplodon are known from the Atlantic and Pacific oceans of South America; these are M. bowdoini (Andrews’ beaked whale), M. densirostris (Blainville’s beaked whale), M. europaeus (Gervais’ beaked whale), M. ginkgodens (Ginkgo-toothed beaked whale), M. grayi (Gray’s beaked whale), M. hectori (Hector’s beaked whale), M. layardii (Strap-toothed beaked whale), M. mirus (True’s beaked whale), M. peruvianus (Pygmy beaked whale), and M. traversii (Spade-toothed whale ; McLeod et al. 2006). Six species of Mesoplodon have been reported in Chile: M. densirostris, M. grayi, M. hectori, M. layardii, M. peruvianus, and M. traversi (Canto and Yáñez 2009; D’Elía et al. 2020). Reports of these species in the Chilean coast are based on less than 20 records, a fact that highlights the importance of documenting any newly emerging record for this genus. These records are based mainly on bone remains (Sielfeld 1979, 1980, 1983; Cárdenas et al. 1986; Canto et al. 1992; Reyes et al. 1995; Sanino et al. 2007) and in only a few cases, on specimens in good condition. Among the latter, there is a male of Strap-toothed beaked whale stranded at Caleta Tortel, Aysén Region in 2019 (personal communication S.E-J.), in addition to the new specimen of M. grayi we are reporting in this work.
Information on Gray´s beaked whales consists of 192 records held in GBIF (2019) worldwide. A single record, not included in GBIF, is known from the South American Pacific Ocean, from the Peruvian coast (Reyes 1990). In Chile, Mann (1958) initially reported this species. Later, Venegas and Sielfeld (1978) and Sielfeld (1979) documented for the first time the presence of this species in the country, based on osteological material found at the Strait of Magellan. So far, these are the only records of this species for Chile concentrated in the southern part of the country.
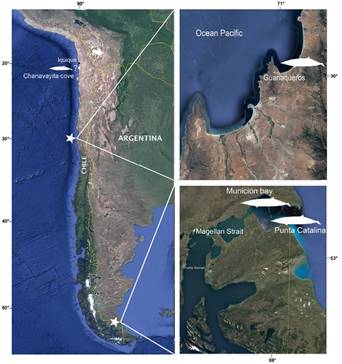
Here we report a new Chilean record for Gray´s beaked whales on the basis a of a specimen initially identified as M. hectori by the Servicio Nacional de Pesca in 2018. This new record, from Guanaqueros beach (Figure 1), confirms the wide distribution of the species in the South-East Pacific, as suggested by MacLeod et al. (2006). The first DNA sequence of any Chilean specimen of this species is also provided. The analysis of skull morphology and coloration suggests that the species presents different morphs, which can be attributed to sexual dimorphism or geographic variation.
Materials and methods
On December 21, 2018, a young female odontocete stranded in the coast of Guanaqueros, Coquimbo Region, Chile, was collected by SERNAPESCA and later transferred to the Museo Nacional de Historia Natural (MNHN), Santiago. A necropsy was performed to determine the possible causes of death. External measurements were taken according to Reyes and Waerebeek (2018), while cranial measurements were taken following Ross (1984). Muscle tissue samples were fixed in 95 % ethanol. Fat samples, the respiratory system, the heart, the uterus and the ovaries were frozen at -18° C. All bone material, as well as the samples, are housed in the Mammal Collection of the MNHN under catalogue number MNHN/MAM 1972.
For the specific determination of specimen MNHN/MAM 1972, comparisons were made with the descriptions and morphometric data of adult specimens of M. hectori, M. peruvianus, M. densirostris, M. bowdoini, M. perrini, M. stejnegeri, M.grayi, and M. europaeus (Ross 1970; Venegas and Sielfeld 1978; Sielfeld 1979; 1983, Lichter 1986; Mead and Baker 1987; Nobuyuki et al. 1987; Mead 1989; Baker and Helden 1999; Norman and Mead 2001; Zerbini and Secchi 2001; Dalebout et al. 2002; Capazzo et al. 2005; Laporta et al. 2005).
In addition, genetic analyses were performed based on a fragment (801 bp) of the mitochondrial gene encoding the cytochrome b (cytb). Total genomic DNA was extracted from a sample of fresh muscle tissue using the Wizard SV Genomic DNA Purification System (Promega ©). The cytb fragment was amplified using primers MVZ 05 and MVZ 16 following the protocol mentioned in Chiquito et al. (2014). The new DNA sequence was edited, including checking for the existence of internal stop codons and changes in the reading frame, using CodonCode (Codon-Code, Dedham, MA, USA). The new DNA sequences were deposited in GenBank under accession number MT813067.
Sequence alignment was done with MAFFT v.7.310 (Katoh and Standley 2013) using L-INS-i as an iterative refinement method (Katho and Toh 2008). Model TIM2+F+I+G4 was selected with IQ-TREE v.1.6.5 (Trifinopoulos et al. 2016). Two phylogenetic methods were used. Maximum Likelihood (ML) analysis was conducted in IQ-TREE with perturbation strength was set to 0.5 and the number of unsuccessful iterations set to 100; nodal support was evaluated by 1,000 ultrafast Bootstrap replicates (BS; Akaike 1973). A Bayesian analysis (BI) was conducted in MrBayes 3.2 (Ronquist and Huelsenbeck 2013) by means of two independent runs, each with five heated and one cold Markov chains and all model parameters estimated in MrBayes. Uniform-interval priors were assumed for all parameters except base composition and substitution model parameters, which considered a Dirichlet prior. Runs lasted 50 million generations, with trees sampled every 1,000 generations. Convergence was checked by plotting log-likelihood values against generation time. The first 25 % of the trees generated were discarded as burned, and only the remaining trees were used to compute a 50 % majority rule consensus tree and to obtain posterior probability (PP) estimates for each clade. Both analyses included sequences of all members of the family Ziphiidae, and Physeter catodon was employed as an outgroup; see supplementary material 1 for the accession numbers.
A list of Chilean strandings and other records involving species of Mesoplodon was compiled from the literature and qualified reports. References to unsupported sightings were excluded.
Results
According to SERNAPESCA, specimen MNHN/MAM 1972 would have died very shortly before stranding. This statement is based on its general good conditions, including coloration and absence of external scars or bruises (Figure 2A-B). The necropsy revealed the skull was severely damaged (see supplementary material 2).
Specimen MNHN/MAM 1972 corresponds to a juvenile female, as cranial sutures are not completely fused, especially the front-supraoccipital one. Furthermore, the skull presents a globose shape at the parietal level, which is characteristic of juvenile cetaceans (Del Castillo 2016). In the nasal region, at the vertex level, a posteroanterior projection of smaller size is observed. The mesorostral canal is cylindrical and does not present ossification like adult specimens of the same species and other species of the genus (Besharse 1971).
Body and skull-mandibular measurements of specimen MNHN/MAM1972 are presented in Table 1. The specimen measured 2.47 m and weighted 185 k. The body has a spindle shape, showing the largest circumference around its midpoint. The beak is short and narrow with the line of the mouth curved shaped (Figure 2B). There is a black patch around the eyes that projects towards the top of the head, in front of the respiratory opening, but with a less marked coloration, tending to grey. The ventral zone of the mandibular (except the area of the beak) and the gular region is greyish-white. The beak is a dark brown that fades slightly towards the melon area where it intersects with grey tones. Behind the respiratory opening, it presents a light pale cream color with a variation of shades in the form of irregular patches. The dorsal area of the body also presents a light beach color with variation in the shades that extend along the entire back. Ventrally, the coloration is greyish white and extends to the caudal peduncle. The dorsal fin is dark grey. The dorsal surface of the pectoral fins has grey color with a variation of shades to darker tones. The caudal lobes in dorsal view are grey, whilst on the ventral side, are light grey.
In the skull the anterior border of the pterygoid sinus reaches the level of the maxillary foramina (= dorsal infraorbital foramina according to Mead and Fordyce 2009). The left premaxillary ridge is not marked and is smaller than the one in the right (Figure 2C, D, E and F). A pair of teeth located in the area of the mandibular symphysis is evident. The jaw is very short compared to adult specimens of the same species (Figure 2G-H). Both the mandibular condyle and coronoid process were not preserved in both mandibular branches.
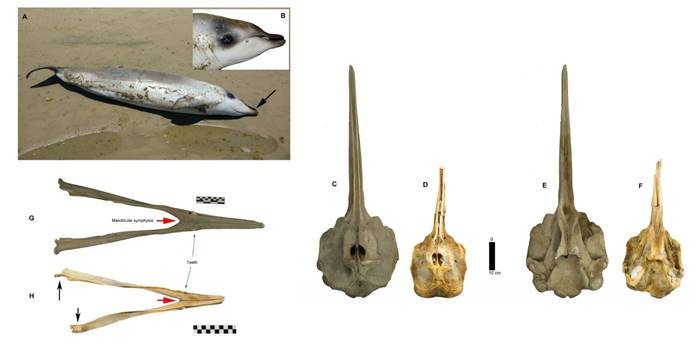
Figure 2 A) View of specimen MNHN / MAM 1972 at its site of stranding on Guanaqueros beach, Coquimbo Region, Chile. The literature mentions a dark brown, dark grey or black colouration pattern on the dorsal surface and paler on the ventral surface, with the tip of the bill being generally white, unlike the rest of the body (Wilson and Mittermeir 2014). Specimen MNHN/MAM 1972 shows a different colour pattern with a dark-coloured peak (see black arrow). The preservation of the colouration stands out because photo was taken only a few hours after the specimen stranded. B). Detail of the head of specimen MNHM / MAN 1972 showing its general colouration and the shape of the beak. C-F) Dorsal and ventral view of the skull of (C and E) an adult female specimen of Mesoplodon grayi (Smithsonian Museum of Natural History Mammal Collection USNM A 49880) and (D and F) specimen MNHN/MAM 1972. G-H) Dorsal view of the mandible of (G) an adult female Mesoplodon grayi (USNM A 49880) and (H) specimen MNHN/MAM 1972, without the mandibular condyle and coronoid process (black arrows). In both specimens, it is observed that the position of the teeth is immediately in front of the mandibular symphysis (red arrows).
The ML and BI analyses showed that the relationship between the members of the family Ziphiidae are mostly unresolved and poorly supported; the genus Mesoplodon was recovered as paraphyletic with respect to Indopacetus pacificus. However, all the species of Mesoplodon were recovered monophyletic and with high support. The sequence obtained for MNHN/MAM 1972 falls in the clade formed by sequences of M. grayi (BS = 99; PP = 1; Figure 3). The sequence obtained from MNHN/MAM 1972 diverges on average from other sequences of Gray´s beaked whales by 1.45 %, while divergence with that of M. perrini is 8.97 % and M. peruvianus 10.71 %. In the sequence distance matrix by species, values ranging between 0.00783 and 0.0225 are observed for four M. grayi sequences (see supplementary material 4). M. grayi form a clade (BS = 95; PP=0.89) with M. peruvianus and M. perrini.
Twenty-two records of the genus Mesoplodon have been reported for Chile, only three of which have been identified as M. grayi, and additionally, a record is indicated on the coast of Iquique for the species (Figure 1, Table 1).
Table 1 Records of species of the genus Mesoplodon along the Chilean coast (1968-2020).
| Specie | Locality | Date | Reference | Comment |
|---|---|---|---|---|
| M.hectori | Windhond bay, Navarino island, Region of Magallanes and Chilean Antarctica | March, 1979 | Sielfeld 1979 | Skull housed in Zoological collection Instituto de la Patagonia (CE-24) |
| M. layardii | Windhond bay, Navarino island, Region of Magallanes and Chilean Antarctica | March, 1979 | Sielfeld 1979 | Skull housed in Zoological collection Instituto de la Patagonia (CE-25) |
| Rio Seco, Punta Arenas, Region of Magallanes and Chilean Antarctica | 1968 | Venegas and Sielfeld 1978 | Picture of specimen housed in files of Instituto de la Patagonia, Universidad de Magallanes | |
| Cabo Espiritu Santo, Tierra del Fuego | February 15, 1978 | Venegas and Sielfeld 1978 | Right tooth housed in Zoological collection Instituto de la Patagonia Universidad de Magallanes (CE-4) | |
| Tortel, Aysen Region | March 20, 2019 | SERNAPESCA 2019 | Tissue sample in housed in mammals collection Museo Nacional de Historia Natural (MNHN/MAM 1973) | |
| M. grayi | Wreck Point, Posesión Strait of Magellan coast | October, 1979 | Sielfeld 1979 | Skull and mandible housed in Zoological collection Instituto de la Patagonia Universidad de Magallanes (CE-26) |
| Wreck Point, Posesión Strait of Magellan coast | December 12, 1979 | Sielfeld 1983 | Skull and mandible housed in Zoological collection Instituto de la Patagonia CZIP 0510. Old number = (CE-28). The collect place is was modified (Erika Mutschke, Universidad de Magallanes) | |
| Catalina Point | May 28, 1978 | Sielfeld 1983 | Skull housed in Zoological collection Instituto de la Patagonia Universidad de Magallanes (CE-35) old number RNP 702 | |
| Strait of Magellan | ||||
| Guanaqueros Coquimbo Region | December 21, 2018 | This work | Specimen housed in Museo Nacional de Historia Natural MNHN/MAM 1972 | |
| 30°11’S 71°24’W | ||||
| M. grayi ? | Chanavayita cove, Iquique Region | April 06, 2002 | SERNAPESCA 2021 Iquique | Stranded one specimen. Identification by Guillermo Guzmán UNAP |
| M. peruvianus | -29° S | February 1998 | Sanino et al. 2007 | Sighting, two specimens |
| -20° 26’ S, -70° 44’ W | December 17, 1997 | Sanino et al. 2007 | Sighting, one specimen | |
| -17° 04’ S, -71° 23’ W | May, 1995 | Sanino et al. 2007 | Skull | |
| M.densirostris | Pargua bay, Los Lagos Region | March, 1980 | Pastene et al.1990 | Stranded one specimen |
| -41° 45’ S, -73° 29’ W | ||||
| Coliumo bay, Concepcion Region 36° -32’ S, -73° 57’ W | June 19, 1999 | Stranded one specimen. | ||
| Vaihu beach Easter island | August 31, 1994 | Aguayo et al. 1998 | Stranded one specimen. The possible cause of death was a shark attack. | |
| Mesoplodon traversii | Juan Fernández island | June 1986 | Val Helden et al. 2002 | Specimen housed in Museo Nacional de Historia Natural MNHN/MAM 1156 |
| Mesoplodon sp. | -32° 30’ S, -88° 42’ W | June, 17, 1995 | Aguayo et al. 1998 | Sighting, one specimen |
| -31° 41’S, -93° 07’ W | June, 18, 1995 | Aguayo et al. 1998 | Sighting, one specimen | |
| -33° 08’ S, -74° 27’ W | July, 08, 1995 | Aguayo et al. 1998 | Sighting, two specimens | |
| -33° 08’ S, 74° 27’ W | September, 15, 1995 | Aguayo et al. 1998 | Sighting, specimen | |
Discussion
Chilean records of Mesoplodon, based on sightings or on strandings, are scarce. Compared to other cetaceans, such as Pseudorca, Tursiops, Lagenorhynchus, Cephalorhynchus, and Phocoena, these records are considerably less frequent (Aguayo 1975). Information compiled by SERNAPESCA (2021) between 2009 and 2021 include 1.093 strandings of cetaceans in Chile, of which only two correspond to species of Ziphiidae. One of the main causes of the low frequency of occurrences is that species of Mesoplodon, and more generally of ziphiids, mainly inhabit the oceanic zone, reaching only occasionally the continental coasts. However, during 2018 and 2020 both sightings and strandings of various species of this family have increased throughout the world (Whitehead et al. 2008; Bernaldo de Quirós et al. 2019; Grove et al. 2020), a pattern that was also observed for cetaceans off the coast of Chile according to records obtained by the Chilean National Fisheries Service (SERNAPESCA 2021) between 2009 and 2021. There is little information associated with the known Chilean records of the six Mesoplodon species that have been recorded. Most of the documented records are based on incomplete bone material, which prevents the collection of data on coloration, sex and other characteristics. As such, the record presented in this study is relevant as it belongs to Gray´s beaked whales. Mann (1958) was one of the first authors to mention the presence of M. grayi in Chile. However, Mann (1958) did not provide any geographic references or other information to support his mention. Therefore, we recommend this record to be ignored until further confirmation becomes available. There is also a mention for Chanavayita beach (Iquique) which is doubtful and should be taken with caution as the putative record is not associated with any preserved specimen. Therefore, only four records of this species are confirmed for Chile.
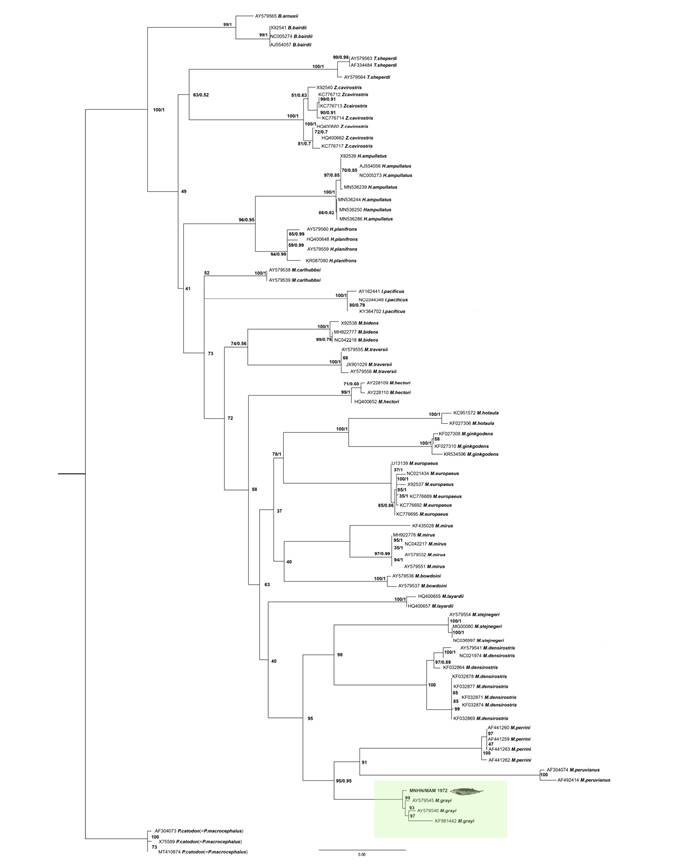
Figure 3 Bayesian phylogenetic tree resulting from the analysis of cytochrome b gene DNA sequences of specimens of Mesoplodon. Numbers at nodes indicate posterior probability (PP; left of the diagonal) and boostrap support (BS; right of the diagonal) values of the adjacent node. The coloured rectangle indicates the position of the specimen MNHN/MAM 1972 within the phylogenetic tree. The GenBank accession numbers of analyzed sequences are included at terminal labels. The terminal corresponding to the new record of M. grayi MNH/MAM 1972 is highlighted in bold.
Based primarily on coloration, SERNAPESCA assigned specimen MNHN/MAM 1972 to M. hectori. The coloration pattern for this species is dark brown, dark grey or black and paler on the ventral surface. The tip of the beak is generally white, unlike the rest of the body (Wilson and Mittermeir 2014). However, specimen MNHN/MAM 1972 presents a different color pattern with a dark colored beak (Figure 2B). The integration of morphological and molecular information allowed us to determine that specimen MNHN/MAM 1972 belongs to Mesoplodon grayi. For this species, the body length varies from 474 to 564 cm in adults and 210 to 242 in newborn calf (Reidenberg and Laitman 2009). Specimen MNHN/MAM1972 presents lower values (247 cm). The beak is long and narrow with an almost straight line of the mouth (Redford and Einsenberg 1992; Wilson and Mittermeir 2014), unlike specimen MNHN/MAM 1972 which has a short beak and a curved buccal line, characteristics that could be attributed to its juvenile condition. The literature reports small teeth at the maxillary level (Von Haast 1876; Sielfeld 1979, 1983; Reyes and Molina 1997), which are also observable in the reported specimen (Figure 2). The skull shows the anterior border of the pterygoid sinus at the level of the maxillary foramen (= dorsal infraorbital foramen according to Mead and Fordyce 2009). The left premaxillary ridge is smaller and less marked than the right one. Finally, mature males have a well-developed pair of teeth in the jaw, while in females these teeth are smaller and not always visible as are covered by the gum (Mead 1989); the latter is the case of MNHN/MAM 1972. The difference in the location of the teeth in the jaw between M. hectori and M. grayi is a distinctive character. In M. hectori, the teeth are located at the tip of the mandible (apical), while in M.grayi, they are immediately anterior to the mandibular symphysis (Reyes and Molina 1997). It is important to mention that position of the teeth in M. grayi varies (Von Haast 1876; Robson 1975; Sielfeld 1979; Reyes 1990). The difference observed by these authors may be due to ontogenetic differences of the species. It should also be considered that there are other sources of phenotypic variation that could explain the distinction of specimen MNHN/MAM 1972. Thompson et al. (2014) mentioned the existence of sexual dimorphism expressed in the skull for this species in two areas of New Zealand, as well as geographic variation. The differences found in specimen MNHN/MAM 1972 may be due to its juvenile condition (Table 2).
Table 2 Measurement of specimen MNHN/MAN 1972 and of other specimens Mesoplodon grayi (in cm).
| MNHN/MAM1972 | Female Mean (Thompson et al. 2014) | CE-26 | |||
|---|---|---|---|---|---|
| Sielfeld 1979 | |||||
| 1 | Condylobasal length | 45.5 | 78.2-90.4 | 73 | |
| 2 | Length of rostrum, tip of beak to line connecting apices of antorbital notches | 25.7 | 53.9-62.9 | --- | |
| 3 | Tip of rostrum to most posterior margin of pterygoid near midline | 34.0 | 62.4-74.5 | --- | |
| 4 | Tip of rostrum to most posterior extension of wing of pterygoid | 35.0 | 70.6-76.8 | --- | |
| 5 | Tip of rostrum to most anterior extension of pterygoid | 24.5 | 45.9-55.7 | --- | |
| 6 | Tip of rostrum to most posterior extension of maxillaries between pterygoids on the palate | 27.5 | 51.1-60.7 | --- | |
| 7 | Tip of rostrum to most posterior extension of maxillary plate | 41.0 | 73.5-83.85 | --- | |
| 8 | Tip of rostrum to anterior margin on superior nares | 30.0 | 59.8-69.6 | --- | |
| 9 | Tip of rostrum to most anterior point on premaxillary crest | 33.6 | 63.9-73.2 | --- | |
| 10 | Tip of rostrum to most posterior extension of temporal fossae | 42.8 | 79.1-89.5 | --- | |
| 11 | Tip of rostrum to most posterior extension of lateral tip of premaxillary crest | 33.7 | --- | --- | |
| 12 | Tip of rostrum to most anterior extension of pterygoid sinus | 24.0 | 49.1-55.7 | --- | |
| 13 | Length of temporal fossa | 7.8 | 8.15-10.83 | --- | |
| 14 | Length of orbit | 7.7 | 8.8-10.9 | 8.7 | |
| 15 | Length of right nasal on vertex of skull | 4.0 | --- | --- | |
| 16 | Length of nasal suture | 3.8 | 2.36 - 4.3 | --- | |
| 17 | Breadth of skull across postorbital process of frontals | 19.8 | --- | ||
| 18 | Breadth of skull across zygomatic process of squamosals | 19.5 | 28.4-31.8 | --- | |
| 19 | Breadth of skull across centres of orbits | 18.2 | --- | ||
| 20 | Least breadth of skull across posterior margins of temporal fossae | 16.0 | --- | ||
| 21 | Greatest span of occipital condyles | --- | --- | ||
| 22 | Greatest width of an occipital condyle | 2.6 | 3.43-4.53 | --- | |
| 23 | Greatest length of an occipital condyle | 5.0 | 1.88-3.36 | --- | |
| 24 | Greatest breadth of foramen mangun | --- | --- | ||
| 25 | Breadht of skull across exoccipitals | 16.4 | 26.6-31.7 | --- | |
| 26 | Breadth of nasal vertex | 4.1 | --- | --- | |
| 27 | Least distance between premaxillary crest | 1.3 | --- | --- | |
| 28 | Greatest extension of right premaxillary posterior of right nasal on vertex skull | 4.7 | --- | --- | |
| 29 | Greatest span of premaxillary crest | 8.6 | 12.12-14.66 | 12.1 | |
| 30 | Least width (strictly transverse) of premaxillae where (and if) they narrow opposite superior nares | 8.6 | --- | --- | |
| 31 | Greatest width of premaxillae anterior to place of measurement N°30 | 5.3 | --- | --- | |
| 32 | Width of premaxillae at midlenght of rostrum | 1.8 | --- | --- | |
| 33 | Width of rostrum in apices of antorbital notches | 12.7 | --- | --- | |
| 34 | Width of rostrum in apices of prominential notches (if any) | ---- | --- | --- | |
| 35 | Greatest width of rostrum at midlength of rostrum | 2.7 | --- | --- | |
| 36 | Greatest depth of rostrum at midlength of rostrum | 2.7 | 3.1-3.9 | --- | |
| 37 | Greatest transverse width of superior nares | --- | --- | --- | |
| 38 | Greatest inside width of inferior nares, at apices of pterygoid notches, on the pterygoids | --- | --- | --- | |
| 39 | Heigth of skull. Distance between vertex of skull and most ventral point on pterygoids | 18.2 | --- | --- | |
| 40 | Greatest width of temporal fossa approximately at right angles to greatest length | 5.3 | --- | --- | |
| 41 | Least distance between (main or anterior) maxillary foramina | 2.0 | --- | --- | |
| 42 | Least distance between premaxillary foramina | 1.4 | --- | --- | |
| 43 | Distance from posterior margin of left maxillary foramen to most anterior extension of left maxillary prominence | 6.5 | --- | --- | |
| 44 | Greatest length of vomer visible at surface of palate | 5.3 | --- | --- | |
| 45 | Amount added to break because of breakage | 1.0 | --- | --- | |
| 46 | Length of tympanic bulla, left | -- | --- | --- | |
| 47 | Length of tympanic bulla, right | 4.9 | --- | --- | |
| Table 2. Continuation | MNHN/MAM1972 | ||||
| 1 | Length of mandible | 38 | |||
| 2 | Greatest length of symphysis | 9,6 | |||
| 3 | Heigth of mandible at coronoid process | 7,8 | |||
| 4 | Outside heigth of mandible at midlength of alveolus | 3,3 | |||
| 5 | Inside heigth of mandible at midlength of alveolus | --- | |||
| 6 | Length from most posterior extension of symphysis to most posterior extension of condyle | --- | |||
| 7 | Length from posterior margin of alveolus to condyle | --- | |||
| 8 | Length of alveolus | 3 | |||
| 9 | Width of alveolus | 0,8 | |||
| 10 | Tip of mandible to alveolus | 8,1 | |||
| 11 | Greatest length of tooth | --- | |||
| 12 | Greatest antero-posterior width of tooth at approximately right angles to long axis of tooth | --- | |||
| 13 | Greatest breadth of tooth | --- |
Worldwide, 158 specimens of Mesoplodon grayi are currently available in collections (Figure 4). These specimens mainly come from three well-defined geographic areas: 1) Australia-Tasmania, which has the highest number of preserved and observed specimens, 2) New Zealand, and 3) the Atlantic coast of South America (GBIF 2019). Only one record is known, from the coast of Peru (Paracas), from the Pacific coast of South America; corresponds to a stranded female of M. grayi identified by external phenotype and skull measurements (Reyes 1990). As such, records on the Pacific coast of South America are scarcely reaching to six with this new Chilean record for M. grayi which is also very valuable as it is the first of a complete specimen from which molecular data are presented.
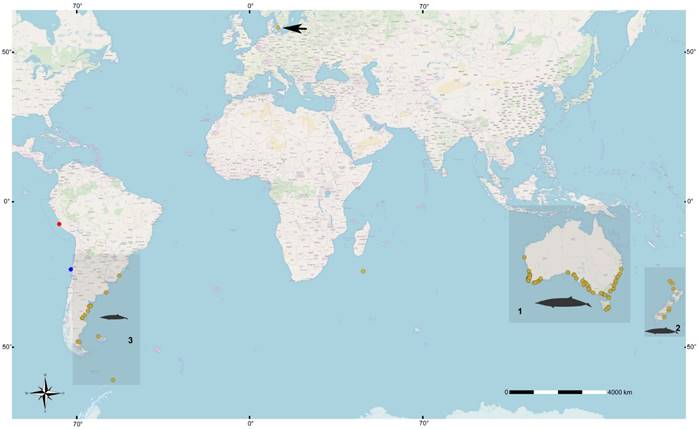
Figure 4 World map showing the distribution of Mesoplodon grayi records. These are concentrated in three main geographical areas: 1) Australia-Tasmania, which presents the most significant number of records, 2) New Zealand; and finally, 3) the Atlantic coast of South America. Note the records on the Pacific coast of South America are limited to two, one in Peru and the other corresponding to the one presented here. The blue circle indicates the new record of M. grayi in Chile (MNHN/MAM 1972); the red circle is the registry in Peru; the yellow circles are the records (strandings and sightings) in other parts of the planet. The black date that indicates the yellow circle in the northern hemisphere, indicates the existence of a specimen deposited in a collection, so it does not represent the distribution of the species











 nova página do texto(beta)
nova página do texto(beta)