Introduction
For small and medium mammals like rodents and lagomorphs, food availability is one of the major limitations for population density (Prevedello et al. 2013). Studies exploring trophic interactions between leporids and other sympatric herbivores, such as livestock, are important to design conservation plans with an adequate livestock management program. It has been widely documented that, in some cases, heavy livestock grazing, and high stocking rates have an adverse impact on the density, distribution and population dynamics of leporids (Gahr 1993; Bock et al. 2006) and other mammals (Cortés-Marcial et al. 2014). Several studies have clearly shown that trophic competition occurs between leporids and other herbivores and ruminants such as cattle (Smith et al. 2004; Young et al. 2005), producing an adverse effect on leporid populations (Hulbert and Andersen 2001). However, certain leporids and livestock can coexist and obtain mutual benefits when stocking rates do not exceed the grazing capacity of their habitat (Karmiris et al. 2005; du Toit 2011). Furthermore, some evidence suggests that livestock grazing, whether by goats, sheep, or cattle, is beneficial to jackrabbits due to the reduction of standing biomass, which is a key factor in habitat suitability (Kuijper et al. 2008; Karmiris and Nastis 2010).
The Tehuantepec jackrabbit (Lepus flavigularis) lives in four disjunct populations within an area of 673 km2 in the surroundings of Laguna Inferior and Laguna Superior in the Tehuantepec Isthmus, Oaxaca, México. This jackrabbit is an endangered species, endemic to Oaxaca, and is currently considered one of the most endangered leporids in the world (SEMARNAT 2010; Lorenzo et al. 2015, 2018; IUCN 2022). L. flavigularis inhabits open grasslands and xeric shrublands with the presence of isolated tree species (Carrillo-Reyes et al. 2012; Lorenzo et al. 2015). Land-use change due to the expansion of human settlements, overgrazing by cattle, and burning of pastures for seasonal agriculture, have reduced and fragmented its habitat. Existing populations are therefore almost entirely isolated and exhibit little genetic variability (Rioja et al. 2011; Lorenzo et al. 2015). A previous study described the diet of L. flavigularis in one locality (Montecillo Santa Cruz) and observed that it feeds mainly on grasses (66.7 % of diet). However, the diet of this species in localities that exhibit different ecological (vegetation associations) and anthropic scenario (production activities), remains unknown. At Santa María del Mar, the jackrabbit shares the grasslands with Bos taurus Zebu breed (treated here as synonym of B. indicus or B. t. indicus), but the trophic interaction between these herbivores is unknown. Because there is dietary overlap between cattle and other leporids like L. californicus, Sylvilagus audubonii (Peña-Neira 1980; Daniel et al. 1993), and Oryctolagus cuniculus (Bonino 2006, 2011), we hypothesize trophic competition between Tehuantepec jackrabbit (L. flavigularis) and cattle (B. taurus). The study aimed not only to determine the seasonal diet of an endangered leporid (L. flavigularis) and cattle, but also to identify possible dietary overlap between these herbivores to better understand this relationship.
Materials and methods
The study area covers an extension of 14 km2 around the locality of Santa María del Mar (16° 14’ 7”, 16° 12’ 46” N and - 94° 53’ 9”, - 94° 48’ 15” W; Figure 1), in the municipality of Juchitán de Zaragoza, in the state of Oaxaca, southern México. It is located in the region of the Tehuantepec Isthmus between a coastal lake (Mar Tileme) and the Pacific Ocean. The town is inhabited by over 800 people whose main productive activities are fishing and cattle production, and occasionally, seasonal agriculture and subsistence hunting (Carrillo-Reyes et al. 2010). The local climate is warm sub-humid with a pronounced dry season, summer rains, and average annual precipitation of 800 mm (Aw0, savanna like). The driest month has less than 60 mm of precipitation and the average annual temperature is 30 °C. The wet season occurs between May and October with a short dry period in August, while the long dry season begins in November and ends in April (García and Comisión Nacional para el Conocimiento y Uso de la Biodiversidad 1998; Rioja-Paradela et al. 2012).
This L. flavigularis population is the most abundant of the four existing; Vargas (2000) recorded 12 individuals/km2 (0.12 individuals/ha) although this value has decreased to 8 individuals/km2 (0.08 individuals/ha; Chacón-Trinidad et al. 2020). The habitat of L. flavigularis is characterized by extensive zones of grassland, dominated by Eragrostis prolifera, Jouvea pilosa and Whalteria preslii (Carrillo-Reyes et al. 2012). Also, isolated individuals of Opuntia tehuantepecana and O. decumbens are present; these areas are grazed by cattle (Carrillo-Reyes et al. 2010). B. taurus and L. flavigularis are the only large and medium herbivores present in this grassland. In the scrubland surrounding the pasture occurs the Eastern cottontail (Sylvilagus floridanus) but does not make use of the grassland; its habitat is limited to the bush, so it does not compete for food or territory with the jackrabbit or the livestock (Rioja-Paradela 2008). A recent study showed that in the grassland association the density of cattle was eight individuals/ha (800 individuals/km2; Chacón-Trinidad et al. 2020), and those cattle grazed in paddocks from 8:00 to 20:00 hrs. There is no rotational grazing; cattle management depends on the availability of "paddocks to lease" and the ability of livestock owners to rent these paddocks.
We made two visits to the study area, one for dry and one for wet season, each lasting 15 days. For vegetation sampling in both seasons, 16 linear transects of 60 m were randomly established with a distance of at least 100 m between them (Carrillo-Reyes et al. 2010). Forage availability was estimated considering vegetation cover as an approximate measure of availability that is compatible with fecal samples at a spatial-temporal scale (Norbury and Sanson 1992; Kufner et al. 2008; Tirado et al. 2012). To record the cover of grasses and herbaceous species along each transect, five circular plots of 1 m2 were established at 10-m intervals along each linear transect, for a total of 80 circular plots. To estimate the shrub cover, one circular plot of 12.6 m2 was established at the center of each linear transect, for a total of 16 circular plots. Calculation of absolute coverage was based on the methodology described by Río-Olague (1999), and relative vegetation cover was calculated using the formula of Franco-López et al. (1989). Significant differences (P < 0.05) in the forage availability between dry and wet season were determined by U Mann-Whitney test (Bauer 1972). For all plant species, samples of leaves and fruits were collected and later processed by the microhistological technique to create a reference collection. This collection was later used to identify the species in the diet of the two herbivores.
During each visit to the study area, we collected L. flavigularis and B. taurus fecal samples. For each species, the freshest excreta were collected along the same transects used for vegetation (16 sampling sites, 1 per vegetation transect), to increase the probability of collecting samples from different individuals. At each transect, random L. flavigularis pellets and B. taurus dung pats were collected and placed in paper bags for transport at ambient temperature. From all fecal samples collected, a total of 20 L. flavigularis random pellets were chosen. Also, for B. taurus the sample was homogenized (the sample was dispersed and mixed homogeneously), and then a random sample of approximately 10 g of excreta was chosen. The samples were then dried at 75 °C for 24 h before subsequent analysis (Bonino 2006; Lorenzo et al. 2011).
The seasonal and annual botanical composition of the diet was determined by microhistological analysis of plant epidermal fragments present in the feces (Peña-Neira and Habib-de Peña 1980; Kufner et al. 2008; Lorenzo et al. 2011). This technique was used because it does not imply disturbing wildlife and no extractions are needed, which is particularly important for a critically endangered species. For each transect and every species, five temporary slides were prepared, for a total of 80 slides per species. To prepare fecal samples for analysis, the material was cleared using a commercial solution of sodium hypochlorite; samples were immersed approximately five minutes in this solution and washed in water for 20 minutes (Kufner et al. 2008). A plastic template with 7 mm diameter and 5 mm thick holes was used to mount the samples. The template was placed on the slides (so that each slide had the same amount of sample), and the holes were filled with the wet material after being washed. A commercial formula of corn syrup was used as mounting medium for temporary slides. After preparation, slides were sealed using transparent glaze and stored to dry for five days. Microphotographs were taken of all slides. On each slide, 20 fields were examined with an optical microscope (objective x40); five separate slides were examined for each sample, with a total of 100 fields per sampling site and a total of 1,600 per species. Slides were examined using randomly generated non-overlapping coordinates. Microscopic identification of species was performed by comparison with the reference collection of microscope slides of plants. Items whose tissue showed at least three diagnostic microhistological elements were identified to species level. Items that did not meet this criterion were not considered. Unidentified fragments were recorded as unidentified monocotyledons or dicotyledons. We recorded the plant species and accumulated frequency (number of fields in which each identified species was recorded). Botanical composition was obtained by transforming frequency to density using the Fracker and Brischle (1944) table (Lorenzo et al. 2011).
Species richness for each season was determined by the number of registered species (Lorenzo et al. 2011). Differences (P < 0.05) in the diet of both species between the dry and wet seasons were determined by U Mann-Whitney test (Bertolino et al. 2009; Desbiez et al. 2009) and corroborated with a Sorensen quantitative test (QS; Morgia and Bassano 2009). The comparison was made by contrasting the density of each plant species in the diet. Niche breadth was assessed using Levins’ measure, B j = 1/Σ pij 2, where pij is the proportion of diet contributed by plant species i on a given mammal species (Levins 1968). This was standardized to a scale of 0 - 1 following B j = (B - 1)/(N - 1) where N is the total number of plant species. The total number of species eaten at least once by at least one herbivore in each season was used to calculate the index (Mishra et al. 2004; Bertolino et al. 2009).
Interspecific trophic relationship was analyzed based on seasonal diet composition. Diet similarity was evaluated with U Mann-Whitney test (Bertolino et al. 2009) and corroborated with a Sorensen test (Morgia and Bassano 2009), contrasting the density of each plant species. Dietary overlap was estimated with the Pianka (1975) index, performing 5,000 Monte Carlo randomizations to determine the difference between the simulated data and the result of overlap (Desbiez et al. 2009).
To determine the relationship between the seasonal diet of each species and the forage availability in the same season the Pianka (1975) index (Kufner et al. 2008) was used. We use vegetation cover as an indicator of forage availability (Kufner et al. 2008). This analysis can indicate the degree of resource selection at a specific time and can corroborate the role of the species as generalist or specialist. All analyses were performed with the software R and RStudio (R Core Team 2020; RStudio Team 2020) and packages "EcoSimR" (Gotelli et al. 2015), “spaa” (Zhang 2013) and “fossil” (Vavrek 2011).
Results
During the dry season, 33 species of non-woody plants (16 grasses and 17 forbs, i. e., non-grass herbaceous plants) and 16 species of woody plants were recorded. In the wet season, 68 species of non-woody plants (18 grasses and 50 forbs) and 22 species of woody plants were recorded. We found a highly significant difference in forage availability between the dry season and the wet season (U Mann-Whitney test, W = 357, P = 0.001; Figure 2).
Twenty-tree plant species were recorded in the diet of L. flavigularis and 29 species were in the diet of B. taurus (Table 1). Both herbivores fed primarily on Poaceae species throughout the year (L. flavigularis, dry = 79.79 %, wet = 91.54 %; B. taurus, dry = 78.02 %, wet = 84.63 %, Figure 3). Less consumed species were forbs (L. flavigularis, dry = 20.21 %, wet = 8.46 %; B. taurus, dry = 14.09 %, wet = 13.90 %) and shrubs (L. flavigularis, dry = 0.00 %, wet= 0.00 %; B. taurus, dry = 1.32 %, wet = 0.83 %).

Figure 2 Availability (%) of plant species by growth form and by season in Santa María del Mar, Oaxaca, México.
The most important items in the diet of L. flavigularis were grasses and forbs, Bouteloua repens (27 %), Stipa eminens (16.87 %) and Digitaria ciliaris (10.28 %), during the dry season, and S. eminens (29.32 %), B. repens (18.55 %) and B. aristidoides (11.38 %) during the wet season. For B. taurus, the most important items were grasses and forbs, B. repens (22.13 %), Eragrostis prolifera (20.43 %) and S. eminens (14.88 %) during the dry season, and B. repens (22.94 %), S. eminens (19.43 %) and E. prolifera (10.18 %) during the wet season.
No significant differences were found in the seasonal composition of the diet of L. flavigularis (W = 911.5, P = 0.78) or B. taurus (W = 893.5, P = 0.91). These results were corroborated by the Sorensen quantitative test, which showed a high similarity between the seasonal diet of L. flavigularis (QS = 0.8540) and that of B. taurus (QS = 0.8957). The trophic niche breadth was low for both species (L. flavigularis, dry = 0.1439, wet = 0.1250; B. taurus, dry = 0.1663, wet = 0.1674).
From 39 species, 12 were consumed by both species during the two seasons. Seasonally, L. flavigularis and B. taurus shared 13 species on both dry and wet seasons (Table 1). This was coincident with dietary overlap analysis; according to the Pianka (1975) index, the dietary overlap between the two species was high for both seasons (dry O jk = 0.7311, wet O jk = 0.8459). We recorded low seasonal use of available plant species for L. flavigularis (dry season O jk = 0.1788; wet season O jk = 0.3102) and for B. taurus (dry O jk = 0.3378; wet O jk = 0.4022).
Table 1 Botanical composition (%) of the diet of L. flavigularis and B. taurus by season. The percentages are presented in parentheses.
| Family | Species | Life form | L. flavigularis | B. taurus | ||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | |||
| Amaranthaceae (7.69 %) | Amaranthus scariosus | Forb | 0.56 | 0 | 0 | 0 |
| Gomphrena globosa | Forb | 0 | 4.04 | 0 | 0 | |
| Gomphrena decumbens | Forb | 0 | 0 | 0 | 0.44 | |
| Asteraceae (5.12 %) | Erigeron oaxacanus | Forb | 6.62 | 2.01 | 1.85 | 2.63 |
| Lactuca intybacea | Forb | 0.79 | 1.34 | 0.12 | 2.28 | |
| Cactaceae (2.56 %) | Opuntia tehuantepecana | Shrub | 0 | 0 | 0.49 | 0 |
| Commelinaceae (2.56 %) | Commelina erecta | Forb | 7.13 | 0 | 0 | 0 |
| Convolvulaceae (2.56 %) | Ipomoea minutiflora | Forb | 0 | 0 | 0.59 | 0.77 |
| Cyperaceae (2.56 %) | Cyperus articulatus | Forb | 4.79 | 0.14 | 0.16 | 0.07 |
| Euphorbiaceae (5.12 %) | Chamaesyce lasiocarpa | Forb | 0 | 0 | 0.4 | 0.12 |
| Dalechampia scandens | Forb | 0 | 0 | 2.39 | 0 | |
| Fabaceae (15.38 %) | Acacia sp. | Shrub | 0 | 0 | 4.79 | 0 |
| Centrosema pascuorum | Forb | 0 | 0 | 0 | 3.56 | |
| Chamaecrista hispidula | Forb | 0 | 0 | 0 | 0.28 | |
| Desmodium barbatum | Forb | 0 | 0.57 | 0 | 0 | |
| Desmanthus virgatus | Shrub | 0 | 0 | 2.61 | 1.48 | |
| Galactia argentea | Forb | 0.08 | 0.35 | 0 | 0 | |
| Gentianaceae (5.12 %) | Eustoma exaltatum | Forb | 0.23 | 0 | 0 | 0 |
| Malvaceae (5.12 %) | Melochia pyramidata | Forb | 0 | 0 | 1.95 | 0.55 |
| . | Forb | 0 | 0 | 4.32 | 0 | |
| Poaceae (41.02 %) | Sorghum halepense | Grass | 0 | 10.64 | 0 | 0 |
| Bouteloua aristidoides | Grass | 10.11 | 11.38 | 1.81 | 8.42 | |
| Bouteloua hirsuta | Grass | 0 | 0 | 0 | 0.04 | |
| Bouteloua repens | Grass | 27 | 18.55 | 22.13 | 22.94 | |
| Bouteloua sp. | Grass | 0 | 0 | 0.26 | 0 | |
| Cenchrus echinatus | Grass | 2.45 | 4.47 | 1.29 | 4.63 | |
| Digitaria ciliaris | Grass | 10.28 | 6.72 | 8.31 | 5.77 | |
| Digitaria filiformis | Grass | 0.29 | 1.37 | 2.43 | 2.66 | |
| Eragrostis glomerata | Grass | 0.48 | 0 | 0 | 0 | |
| Eragrostis prolifera | Grass | 2.24 | 5.01 | 20.47 | 10.18 | |
| Grass - Unidentified sp1 | Grass | 3.07 | 0.97 | 0.31 | 2.19 | |
| Hilaria belangeri | Grass | 1.63 | 1.06 | 1.62 | 2.85 | |
| Jouvea pilosa | Grass | 0.54 | 0.8 | 4.48 | 1.87 | |
| Muhlenbergia sp. | Grass | 2.47 | 0 | 0 | 0 | |
| Paspalum prostatum | Grass | 0 | 0 | 0 | 3.68 | |
| Stipa eminens | Grass | 16.87 | 29.32 | 14.88 | 19.43 | |
| Stipa sp. | Grass | 2.37 | 1.24 | 0 | 0 | |
| Solanaceae (5.12 %) | Hydrolea spinosa | Forb | 0 | 0 | 2.11 | 3.17 |
| Verbenaceae (5.12 %) | Phyla nodiflora | Forb | 0 | 0 | 0.19 | 0.02 |
Discussion
Results indicate that L. flavigularis and B. taurus exploited a relatively narrow variety of available species of plants, suggesting that both herbivores are selective, showing a preference for Poaceae species throughout the year despite the significant difference in the availability of plant species between the dry and wet season. The Poaceae preference of L. flavigularis is consistent with the findings of Lorenzo et al. (2011) in another isolated L. flavigularis population and with other leporid diet studies (López-Cortés et al. 2007; Karmiris and Nastis 2010; Kontsiotis et al. 2011; Ge et al. 2013; Freschi et al. 2014, 2015; Luna-Casanova et al. 2016). However, we found that some species of plants consumed by L. flavigularis in Santa Maria represent new records for this leporid. The Poaceae preference of B. taurus is also consistent with previous studies in other localities (Quinteros et al. 2013).
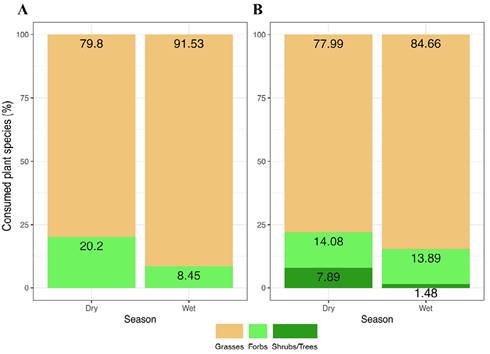
Figure 3 Percentage of consumed plant species (%) by growth form and by season in Santa María del Mar, Oaxaca, México. A: L. flavigularis, B: Cattle.
These results are also related to the plant species availability. The study area is located in a low precipitation region from the Tehuantepec Isthmus, with severe drought from November to April. Despite the fact that grasses have low protein content in comparison to other species (Codron et al. 2007), and contain abrasive silica (Sanson et al. 2007), these species are more resistant to drought than ephemeral or annual forbs (Tilman and Downing 1994) which may cause that grass availability is high year-round. Also, Poaceae species distribute in open areas (Gordon and Prins 2008), which is one of the main characteristics of grassland habitat in the Tehuantepec Isthmus (Farías and Fuller 2009; Rioja et al. 2011). In agreement with these findings, it has been reported that L. flavigularis prefer grassland over other available habitats because, besides providing food, it represents a suitable place to carry out its activities of resting, socialization and reproduction (Farías and Fuller 2009; Rioja et al. 2011; Carrillo-Reyes et al. 2012; Lorenzo et al. 2015; Luna-Casanova et al. 2016).
Studies show the clear occurrence of trophic competition between leporids and other herbivores and ruminants such as cattle (Smith et al. 2004, Young et al. 2005), producing an adverse effect on leporid populations (Hulbert and Andersen 2001). In our study, Lepus flavigularis and B. taurus had a low trophic niche breadth and high dietary overlap, which indicates a high level of resource (trophic) competition that could adversely affect the L. flavigularis population. Other studies state that L. flavigularis prefers to establish feeding, resting, and breeding sites in pastures with the presence of B. taurus (Carrillo-Reyes et al. 2010, 2012; Rioja et al. 2011; Luna-Casanova et al. 2016). It is likely that the presence of B. taurus influence the existence of certain plant species that could serve as a forage resource for L. flavigularis, through the establishment of seedlings from B. taurus feces (Kuijper et al. 2008). Furthermore, B. taurus grazing reduce height, cover, and density of vegetation (Smith et al. 2005; Karmiris and Nastis 2010; Rioja et al. 2011). As Farías and Fuller (2009) stated, open grasslands with scattered trees and shrubs can help L. flavigularis to avoid predators like Canis latrans. Therefore, selection of areas with low vegetation cover may reduce the energy spent in monitoring its habitat, which favors the investment of more time in foraging (Karmiris and Nastis 2010; Rioja et al. 2011; Luna-Casanova et al. 2016). According to previous literature, we expect that jackrabbits benefit from cattle presence, as long as the stocking rate does not increase current levels. Research suggests that dietary partition between grazing herbivores is a common phenomenon and that leporids and cattle can coexist and obtain mutual benefits while not exceeding grazing capacity (Karmiris et al. 2005; Karmiris and Nastis 2010). Over long periods, livestock can even improve forage quality and foraging efficiency, facilitating food availability for leporids (Kuijper et al. 2008; du Toit 2011).
Low trophic niche breadth and high dietary overlap between L. flavigularis and B. taurus, combined with a low seasonal relationship between food availability and diet, suggest that forage resources competition is occurring in this system. Exotic species such as S. eminens and D. ciliaris, and a native species such as B. repens, were highly consumed by both species throughout the year. It is likely that these plant species remain permanently available to both herbivores, either as fresh or dry forage, due to its high silica content and their ability to survive in areas with low water content. On the other hand, certain species were consumed in a certain season by some species. For example, C. articulatus and C. erecta were consumed by L. flavigularis mainly during the dry season, while species S. halepense was consumed during the wet season. We believe that the mobility capacity of the species partly determined these differences. Cattle are restricted to using the resources available in the pastures and roads in which they are kept, while the hare can use almost the entire available area. Hence, it also has permanent or temporal access to all available plant species.
In conclusion, our results show trophic competition between native and no-native herbivorous species. However, we suggest that both species can coexist as long as the site's carrying capacity is not exceeded. On the one hand, although both species feed mainly on grasses, these plant species are the ones that persist throughout the year, even in drought conditions. Additionally, cattle have limited movement to pastures, while L. flavigularis can move freely throughout its range. Also, cattle grazing reduces vegetation's height, cover, and density, facilitating L. flavigularis to avoid potential predators. Overall, cattle management at moderate stocking densities, can be compatible with the subsistence of L. flavigularis populations.











 nueva página del texto (beta)
nueva página del texto (beta)