Introduction
Migration, an important life history trait for many species, allows individuals to spatially and temporally exploit changing environments, including ephemeral food sources. Long-range movements are exhibited by several mammals, including some ungulates, pinnipeds, cetaceans, and bats (Dingle 2014). With over 1,400 extant species (Simmons and Cirranello 2020), bats are one of the most diverse groups of mammals in the world. Despite their recognized importance in community ecology as seed dispersers, pollinators, and suppressors of insect populations, many aspects of their basic biology remain unknown due to the difficulties of studying nocturnal, flying organisms (Frick et al. 2020). Adding to their complexity, reproductive cycles of bats can vary within the same family and even within the same genus at similar latitudes making generalizations difficult (Racey 1982). To assist with bat conservation strategies and planning, studies should aim to increase our understanding of bat reproductive ecology (Racey and Entwistle 2000), including the effect of mating opportunities on the distributions of migratory bats.
Among North American bats, migratory species include temperate bats (many vespertilionids) that move seasonally between hibernation roosts and breeding habitats, temperate species (mostly tree-roosting bats) that migrate south for the winter, and tropical bats that move north to follow food resources and/or give birth (McNab 1982). Long-nosed bats, Leptonycteris spp. (Chiroptera: Phyllostomidae: Glossophaginae), are tropical migrants that rely on the availability of flowering plants. Their migration follows nectar corridors of flowering columnar cacti and paniculate Agave spp. between seasonal roosts (Fleming et al. 1993; Burke et al. 2019), and Leptonycteris spp. are among the most important nocturnal pollinators for many of these species (Valiente-Banuet et al. 1996; Peñalba et al. 2006; Rocha et al. 2006). Observations of low Leptonycteris densities may be caused by population declines due to habitat destruction or natural variation in flowering plant density (Moreno-Valdez et al. 2000), thus requiring a spatial and temporal understanding of bat and plant populations across their range.
Due to current technology limitations, most long-nosed bat movements are inferred from direct observations, passive integrated transponder (PIT) tag detections (Frick et al. 2018), and genetic studies (Wilkinson and Fleming 1996; Menchaca et al. 2020), which led to the discovery that only some populations of the lesser long-nosed bat (Leptonycteris yerbabuenae) migrate. Of the populations dwelling in Mexico, some females are resident to central and southern Mexico, where they form maternity roosts in the winter. Other females migrate between winter roosts in central Mexico and summer maternity roosts in northern Mexico and the southwestern United States (Nassar et al. 2016). For both migratory and resident populations, sexually active males of L. yerbabuenae may form an odoriferous dorsal patch during the mating season, created by a smearing behavior in which saliva, urogenital fluids, and anal secretions are spread over the interscapular dorsal region, similar to the behavior displayed by reproductive males of the Curaçaoan long-nosed bat (Leptonycteris curasoae) in Venezuela (Muñoz-Romo et al. 2011a). Indeed, one observation of a L. yerbabuenae male displaying a similar behavior supports this hypothesis (Laverty and Stoner 2022). Males with developed dorsal patches (i. e., bare dorsal skin with sticky fur; Frick et al. 2018) have larger testes (Rincón-Vargas et al. 2013), and may have lower ectoparasite loads as noted by studies of L. curasoae (Muñoz-Romo and Kunz 2009; Muñoz-Romo et al. 2011b). Together, this suggests that dorsal patches may influence female mate choice; however, other potential explanations have not yet been evaluated.
Due to the differences in migratory behavior among L. yerbabuenae populations combined with reproductive asynchrony, we asked how does the phenology of male reproductive status, specifically the presence of dorsal patches, vary temporally across the species’ range? By identifying where and when mating may occur based on distributions of males with dorsal patches, this study furthers our understanding of the reproductive ecology of L. yerbabuenae and highlights potential mating roosts or regions. We also document the first observations of dorsal patches at the northern extent of L. yerbabuenae’s migratory range and discuss the conservation implications of these findings.
Materials and methods
Study species. The pattern of reproduction of Leptonycteris yerbabuenae has been described as either bimodal polyestrous and monoestrous, but individuals are thought to mate only once per year (Ceballos et al. 1997; Rojas-Martínez et al. 1999; Stoner et al. 2003; Cole and Wilson 2006). The timing of reproduction varies across the species’ range such that females either give birth in the winter or in the spring (Rojas-Martínez et al. 1999; Menchaca et al. 2020). Those that give birth in the winter are residents of central and southern Mexico, and may remain in a single roost year-round (Galindo et al. 2004), or move between roosts seasonally (e. g., altitudinal movements due to food availability; Ceballos et al. 1997; Herrera 1997; Stoner et al. 2003). Females that give birth in the spring are believed to mate in the fall or winter in central Mexico, and migrate in the spring along the Pacific Coast to maternity roosts in northern Mexico and southern Arizona to give birth (Cockrum 1991; Ceballos et al. 1997; Rojas-Martínez et al. 1999; Stoner et al. 2003). Those arriving in the southwestern United States later in the summer fly along the foothills of the Sierra Madre Mountains and may travel from as far south as Jalisco (Wilkinson and Fleming 1996). Occupancy of L. yerbabuenae in southeastern Arizona and southwestern New Mexico generally peaks from mid-August to mid-September and then dwindles by early October when most individuals are thought to have returned south to Mexico (Cockrum 1991; Bogan et al. 2017). Occasionally, small groups of L. yerbabuenae are found beyond October in Arizona (US Fish and Wildlife Service 2016; Menchaca et al. 2020), but it is not known if these individuals survive the winter (S. Wolf, pers. comm.).
While L. yerbabuenae was originally classified as endangered at the northern extent of its range in the late 1980s (Cole and Wilson 2006), the species was removed from the Endangered Species List in the United States in 2018, following its removal from threatened status in México in 2013 (Frick et al. 2018). It is one of three species of nectar-feeding bats-along with the Mexican long-nosed bat (Leptonycteris nivalis) and the Mexican long-tongued bat (Choeronycteris mexicana)-that migrate seasonally from Mexico to the southwestern United States along corridors of ephemeral flowers of cacti and Agave spp. (Bogan et al. 2017; Burke et al. 2019). These bat species are thought to benefit the tequila and mezcal industries by enhancing the genetic diversity of Agave spp. through cross-pollination (Trejo-Salazar et al. 2016).
Study site. Our fieldwork focuses on the migratory populations of L. yerbabuenae at the northern extent of its range. We specifically study those bats inhabiting a cave in the Big Hatchet Mountains in New Mexico, which harbors the easternmost distribution of L. yerbabuenae in the United States (Bogan et al. 2017). Leptonycteris nivalis and L. yerbabuenae in this region are believed to mostly feed on nectar and pollen in the Animas Mountains, requiring bats in the Big Hatchet Mountains roost to commute >20 km in each direction across the Playas Valley to reach this foraging area (Bogan et al. 2017). While the diet of L. yerbabuenae includes flowering cacti in Arizona, the main food source in southwestern New Mexico appears to be limited to Agave spp. (mostly Agave palmeri; Ober and Steidl 2004; Scott 2004). This region is comprised of semidesert grasslands interspersed at higher elevations with patches of Madrean evergreen woodland (alligator bark juniper, piñon, Chihuahua pine, and species of oak), lower interior chaparral (manzanita, mountain mahogany), and interior southwest riparian deciduous forest (sycamore, cottonwood, and rabbitbrush-Apache plume; Brown 1994).
Bat sampling. We captured individuals of L. yerbabuenae in the Big Hatchet Mountains during their seasonal occupancy in 2019, for a total of six nights of sampling between 13 July and 12 September 2019. To capture bats, we placed a 12 m long by 2.6 m high mist net (38-mm mesh; Avinet Inc., Dryden, NY) just downhill of the main entrance of the cave opening. Mist nets were opened at sunset (i. e., at 2030 h in July or as early as 1940 h in September), but the amount of time the net remained open each night was dependent on bat activity and varied across our sampling period (median = 3.84 h/night, range = 3.28 - 6.47 h/night, total = 26.62 h). We scanned for bats in the net every five min, untangling captured individuals that were then temporarily stored in cotton bags until they could be processed. For each individual, we recorded age, sex, reproductive condition, forearm length, and body mass. We determined the age of individuals (i. e., juveniles or adults) based on the relative ossification of the metacarpal-phalangeal joint in the wings (Brunet-Rossinni and Wilkinson 2009). Females were classified as non-pregnant, pregnant, lactating, or post-lactating by abdominal palpation and nipple examination (Racey 2009). Reproductive condition of adult males was determined by examining if the testes were swollen and distended, and if an individual’s interscapular dorsal region presented a dorsal patch (Figure 1). For those individuals without a dorsal patch or wing damage, we inserted a PIT tag subcutaneously between the shoulder blades as part of an on-going migration study. All bats were released at the capture location within 30 min of capture. Field protocols were approved prior to implementation by Colorado State University’s Institutional Animal Care and Use Committee (Protocol #19-8891A), the New Mexico Department of Game & Fish (Permit #3611), and the U.S. Fish & Wildlife Service (Permit #TE63195B-0).
Literature review. In addition to our field study, we searched for peer-reviewed publications that included information on the dorsal patch of male Leptonycteris spp. On 27 August 2020, we used Google Scholar to search for articles published through August 2020 including the keywords “Leptonycteris” and “male” that also included the term “dorsal patch” or “sebaceous patch.” We similarly conducted a search on Web of Science on 27 August 2020 using the topic search TS = Leptonycteris AND male AND (“dorsal patch” OR “sebaceous patch”).
To supplement data at the northern extent of L. yerbabuenae’s range, we also searched through grey literature materials and contacted various researchers in New Mexico and Arizona for observations of reproductive males with dorsal patches.

Figure 1 Photographs of new observations of male Leptonycteris yerbabuenae individuals with dorsal patches in the southwestern United States. Images include (a) a male with a developed dorsal patch at the Big Hatchet Mountains roost, New Mexico in July 2019, (b) a male with a developed dorsal patch at a hummingbird feeder near Hilltop Mine, Arizona in July 2013, and (c) a male with a recovering dorsal patch at the Patagonia bat cave, Arizona in August 2008. Photographs by (a) Theresa Laverty, (b) Joseph Danielson, and (c) Debbie Buecher.
Results
New observations of dorsal patches in New Mexico and Arizona. Between July and September 2019, we captured a total of 55 L. yerbabuenae, which were comprised of 33 adults (9 males and 24 females) and 22 juveniles (5 males and 17 females) in the Big Hatchet Mountains. Three (33 %) of the adult males were identified as reproductively active due to the presence of swollen testes and developed dorsal patches (i. e., bare dorsal skin with sticky fur; Figure 1 and Table 1). A reproductively active male with a forearm length of 55.4 mm and a weight of 29.0 g was captured at 2158 h on 25 July 2019 and a wing punch was taken. Two different males were captured at 2339 h on 13 August 2019 and 0042 h on 14 August 2019. While no marking techniques were used on these latter individuals, they differed in forearm length (54.8 and 56.0 mm), weight (23.5 and 27.5 g), and the appearance of the dorsal patch (i. e., one individual had more bare skin exposed). Since neither of these individuals presented a scar on their wings where the wing punch was taken from the first male and they all differed in forearm lengths and body mass, we could confidently assume these were 3 different adult males (Figure 2).
Three separate studies on L. yerbabuenae in Arizona and one recent study in New Mexico also reported observations of dorsal patches (Figure 2 and Table 1), and three of those observations were confirmed through photographs (Figure 1). Males with developed dorsal patches were captured at the Hilltop Mine (June 2006 or 2007; D. Dalton, pers. comm.) and at a hummingbird feeder located at a residence near the mine (July 2013; J. Danielson and K. Ekholm, pers. comm.). In late August 2008, a male with a recovering dorsal patch (i. e., bare dorsal skin with regenerating fur) was captured at a roost near Patagonia, Arizona (D. Buecher and J. Ramirez, pers. comm.). More recently, another male with a recovering dorsal patch was captured in mid-September 2021 at a hummingbird feeder near Silver City, New Mexico (M. Davies and R. Burke, pers. comm.).
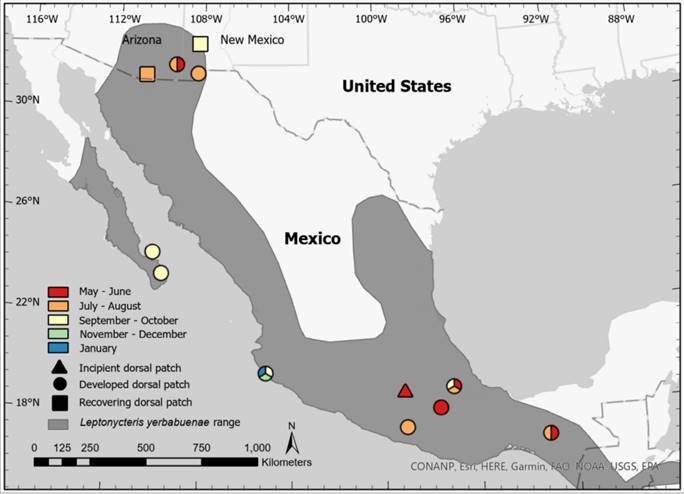
Figure 2 Map displaying locations and timing of observations of male Leptonycteris yerbabuenae with incipient (triangle), developed (circle), and recovering (square) dorsal patches. Sites with observations spanning multiple months are represented by multiple colors. Polygon data obtained from http://www.iucnredlist.org/
Published observations of dorsal patches from other roosts. Our literature review using Google Scholar resulted in 29 publications: 20 peer-reviewed articles, 3 book chapters, 2 independent study reports, 1 Ph.D. dissertation, 1 M.Sc. thesis, and 1 preprint. We found 9 peer-reviewed articles using Web of Science, all of which were also identified by Google Scholar. Of the 29 publications, only 14 were primary literature studies that sampled Leptonycteris spp. in the field. Reproductive males of L. nivalis do not develop dorsal patches as documented by a study that included observations from several mating seasons at the only known mating roost for the species, Cueva del Diablo near Tepoztlan, Morelos, central Mexico (Nassar et al. 2016). Dorsal patches do occur, however, in both L. curasoae and L. yerbabuenae and were first described for both species by Nassar et al. (2008). For L. curasoae, males with dorsal patches have been observed during November and December in Guano Cave and Piedra Honda Cave, Paraguaná Peninsula, Falcón State, Venezuela (Nassar et al. 2008; Muñoz-Romo and Kunz 2009; Muñoz-Romo et al. 2011b; Muñoz-Romo et al. 2011a; Muñoz-Romo et al. 2012). The 8 remaining publications in addition to Nassar et al. (2008) described dorsal patches of male L. yerbabuenae in Mexican roosts (Table 1), where the phenology of dorsal patches-like mating seasons-appears to be asynchronous (Figure 2). Dorsal patches were documented between May and September at southern roosts, between September and January in Chamela, Jalisco, and between September and October at roosts on the southern Baja peninsula.
Table 1 The location, timing, and prevalence of male dorsal patches in Leptonycteris yerbabuenae.
| Location | Timing of dorsal patch | Percentage of males with dorsal patch | Reference |
|---|---|---|---|
| Big Hatchet Mountains, New Mexico, USA | July - August 2019 | 33.3 % (n = 3/9) | This study |
| Hummingbird feeder near Silver City, New Mexico, USA | mid-September 2021 | 20.0 % (n = 1/5) with a recovering dorsal patcha | M. L. Davies & R.A. Burke, pers. comm. |
| Hilltop Mine, Arizona, USA | June 2006 or 2007 | 20 to 30 males, most with dorsal patchesb | D. C. Dalton, pers. comm. |
| Hummingbird feeder near Hilltop Mine, Arizona, USA | July 2013 | 1 male with dorsal patchc | J. R. Danielson & K. L. Ekholm, pers. comm. |
| Patagonia, Arizona, USA | late August 2008 | 1 male with recovering dorsal patchd | J. Ramirez & D. C. Buecher, pers. comm. |
| Chivato, southern Baja California penísula, México | September 2017 | 61.1 % (n = 11/18) | (Frick et al. 2018) |
| Las Cuevas, southern Baja California penísula, México | early October 2013 | 90.0 % (n = 9/10) | (Frick et al. 2018) |
| Chamela Bay, Jalisco, Mexico | November 2002 | 24.2 % (n = 16/66) | (Nassar et al. 2008) |
| December 2002 | 35.1 % (n = 27/77) | (Nassar et al. 2008) | |
| September 2003 | 35.5 % (n = 22/62) | (Nassar et al. 2008) | |
| October 2003 | 50.0 % (n = 24/48) | (Nassar et al. 2008) | |
| October 2008 -January 2009 | 22.2 % (n = 26/117) | (Rincón-Vargas et al. 2013) | |
| January 2019 | 20 of 33 males were reproductivee | (Zamora-Mejías et al. 2020) | |
| Tlilapan, Veracruz, México | June - September 2011 and 2012 | 38.9 % (n = 7/18) | (Ramírez Hernández and Herrera 2016) |
| El Salitre, Morelos, México | June 2019 | 15 of 28 males were reproductive with incipient patchese | (Zamora-Mejías et al. 2020) |
| Colotlipa, Guerrero, México | July 2017 | 18 of 25 males were reproductivee | (Zamora-Mejías et al. 2020) |
| San Juan Noxchitlán, Oaxaca, México | June 2015 | 17 males captured, but only 11 used for DNA extractionf | (Gaona et al. 2016; Gaona et al. 2019a; Gaona et al. 2019b) |
| Los Laguitos, Chiapas, México | May - August 2009 | 61.9 % (n = 13/21) in May diminished to 11.7 % (n = 2/17) in August | (Martínez-Coronel et al. 2017) |
a Correspondence including photographs with researchers Mallory L. Davies and Rachel A. Burke confirmed that a male was captured with a recovering dorsal patch (i. e., new hair growth present) at a hummingbird feeder at a private residence in mid-September 2021. There was a suspected second male with a recovering dorsal patch, but unfortunately no photograph was taken.
b Correspondence with researcher David C. Dalton indicated that all of the 20-30 individuals captured were adult males. Most, if not all, had a dorsal patch. In some cases, the dorsal patches were mostly healed and were partially re-furred. In other cases, the dorsal patches were still open wounds that looked very fresh.
c Correspondence including photographs with researchers Joseph R. Danielson and Kelsey L. Ekholm confirmed that a male was captured with a dorsal patch at a hummingbird feeder at a private residence in July 2013.
d Correspondence including photographs with researchers Judith Ramirez and Debbie C. Buecher confirmed that a male was captured with a recovering dorsal patch (i. e., new hair growth was present) in late August 2008.
e Authors did not state in the publication how many reproductively active males had a dorsal patch. Correspondence with Daniel Zamora-Mejías indicated the presence of males with dorsal patches as well as observed copulations during these time periods with the exception of El Salitre (i. e., where incipient dorsal patches were observed, and it was assumed mating would begin in the upcoming weeks).
f Authors did not state in these publications how many males they captured without a dorsal patch. Correspondence with Osiris Gaona indicated that at least 50 % of the males captures in the Obispos cave presented a dorsal patch.
Discussion
Much of what is already known about the reproductive ecology of L. yerbabuenae is derived from research in Mexico. Reproductive males with dorsal patches have been documented at several mating roosts in Mexico since first being documented in the literature by Nassar et al. (2008). Due to the individual variation in the timing of dorsal patches throughout the species’ range, more longitudinal studies are needed to identify periods in which dorsal patches are present and when mating occurs (e. g.,Rincón-Vargas et al. 2013; Frick et al. 2018). Thus far, these odoriferous patches have only been found in males of L. curasoae and L. yerbabuenae during their mating seasons. Although dorsal patches do not form in L. nivalis (Nassar et al. 2016), they likely play an important role in female mate choice for L. curasoae (Muñoz-Romo and Kunz 2009; Muñoz-Romo et al. 2011a). Dorsal patches could have a similar function in L. yerbabuenae since copulations observed in western central Mexico (Chamela, Jalisco) have only included male individuals with developed dorsal patches (i. e., bare dorsal skin with sticky fur; Laverty and Stoner 2022).
Given the important functions that mating and maternity roosts play in bat population ecology, these roosts are often the focus of conservation efforts. Prior to this study, roosts of L. yerbabuenae in the southwestern United States were thought to function as maternity roosts and transient roosts (i. e., where females and volant young feed before migrating south; Bogan et al. 2017). Through our fieldwork and communication with other researchers in New Mexico and Arizona, we report occasional sightings of developed or recovering dorsal patches (i. e., bare dorsal skin with sticky or regenerating fur, respectively) at the northern extent of L. yerbabuenae’s range, suggesting that males may be seeking out mating opportunities in the southwestern United States between June and September. If so, one or more transient roosts may also function as mating roosts and harbor additional conservation value. Future studies should confirm if L. yerbabuenae breeds in the southwestern United States. Ideally, this would involve researchers briefly visiting roosts during their periods of occupancy to scan for copulating L. yerbabuenae. While netting bats, reproductive males have historically been identified by observing swollen and distended testes, but we recommend that all future studies of L. yerbabuenae measure the external testes with calipers to confirm they meet a minimum size corresponding to complete spermatogenesis (e. g., 48.2 mm2 for L. yerbabuenae in Chamela; Rincón-Vargas et al. 2013) to relate dorsal patch presence with reproductive activity. Prior to the discovery of dorsal patches, Cockrum and Ordway (1959) reported gravid female L. yerbabuenae and males with enlarged testes in mid-August 1955 at a mine near Paradise, Arizona, which suggests that reproductively active males may not be a new occurrence in the United States. While further research is needed exploring the functional role of dorsal patches, all known mating roosts of L. yerbabuenae contain males presenting dorsal patches (e. g.,Rincón-Vargas et al. 2013; Frick et al. 2018; Laverty and Stoner 2022). Therefore, our study highlights how the reproductive status and presence of dorsal patches in male L. yerbabuenae may inform research priorities and aid in the identification of mating roosts.
The role of migration in determining the reproductive status of L. yerbabuenae remains largely unknown. Research in Chamela, Jalisco, found spermatogenesis (i. e., the process of sperm cell development) and the presence of dorsal patches in L. yerbabuenae to occur during peak food availability and the return of migratory females to the region (Rincón-Vargas et al. 2013). However, males can be captured year-round at this study site (Stoner et al. 2003). Roosts in the southwestern United States, on the other hand, are largely unoccupied and food is not available for nectarivorous bats from roughly November to May (Cockrum 1991; Bogan et al. 2017). Given the presumed energetic costs of developing a dorsal patch and mating (Muñoz-Romo and Kunz 2009; Rincón-Vargas et al. 2013), particularly after migrating from and before migrating to unknown roosts in Mexico, we did not expect to find males with dorsal patches that could potentially be seeking out mating opportunities in the southwestern United States. Therefore, our research echoes other studies citing the need for more information on the movements of male L. yerbabuenae (Cockrum 1991; Menchaca et al. 2020). While we do not encourage PIT tagging males with dorsal patches, we recommend future studies mark these males (e. g., with a wing punch or banding) to ensure accurate counts of individuals with dorsal patches at a roost. Males without dorsal patches should continue to be PIT tagged. Until more information is known on the movements of males and until roosts are thoroughly searched for copulatory behaviors, we cannot confirm the presence of a mating roost in the southwestern United States, but the presence of dorsal patches at transient roosts at the northern extent of the species’ range demands further investigation into this matter.











 nova página do texto(beta)
nova página do texto(beta)


