Introduction
Insectivorous and arthropodophagous bats represent about 70 % of the world’s Chiropteran diversity, and more than half of its Neotropical diversity (Fenton and Simmons 2014). Their eating habits highlight their importance as crop pest controllers and stabilizers of arthropod populations (Boyles et al. 2011; Boyles et al. 2013). However, little is known about the feeding habits of insular arthropodophagous and insectivorous bat species, which, unlike their mainland conspecifics, have limited resources that depend on the availability and population dynamics of the island’s exclusive invertebrates, as well as on the competition with sympatric species (Sedlock et al. 2014). This means that island bats must adapt to the restrictions imposed by their environment to survive (McNab 2010). Some bat trophic strategies reported on islands are: 1) opting for a generalist diet (Razakarivony et al. 2005; Rakotoarivelo et al. 2007; Racey et al. 2010), 2) resource partitioning to reduce competition (Zhang et al. 2005; Fukui et al. 2009; Rolfe et al. 2014), and 3) modifying their foraging periods according to the activity patterns of their prey (Bradbury and Vehrencamp 1976; Pavey et al. 2001).
The lesser sac-winged bat, Saccopteryx leptura, is a small insectivorous bat that is widely distributed in the new world tropics, mainly in the intertropical zone. This species can live in a wide range of habitats, from preserved tropical forests to urban areas, and there are even populations on islands (Bradbury and Emmons 1974; Cadena et al. 1990; Vivas-Toro and Murillo-García 2019). Knowledge about S. leptura is scarce, including that concerning its feeding habits. Bradbury and Vehrencamp (1976) made an approximation of S. leptura’s diet composition based on analyses of other emballonurid sympatric species, which suggested they have a diet mainly consisting of Coleoptera and Diptera, with lower consumption of Lepidoptera. However, there are two studies (albeit consisting of few individuals or samples) that propose Hymenoptera as the most consumed order (Nogueira et al. 2002; Cruz-Parrado et al. 2018). Although these studies provide valuable contributions, they still represent first data regarding the diet of S. leptura.
Interestingly, S. leptura has shown a positive response to moonlight intensity, implying higher activity at brighter nights (Appel et al. 2017, 2019). Furthermore, this species has also been reported foraging during the day on islands such as Trinidad (Bradbury and Emmons 1974) and Gorgona (Vivas-Toro and Murillo-García 2020), generating more questions concerning S. leptura feeding habits and what they eat during those time frames. Frequent daytime activity of insectivorous bats is unusual (Russo et al. 2011a). Except for the early forager Pipistrellus pygmaeus from central Italy (Russo et al. 2011a), to date, all species reported exhibiting this behavior reside on islands (Bradbury and Emmons 1974; Moore 1975; Russo et al. 2011a; Russo et al. 2011b; Chua and Aziz 2019; Vivas-Toro and Murillo-García 2020). Among the common patterns displayed by these bats during the day are the tendency to feed mainly in closed canopy sites with abundant insect prey, and a decreasing activity with increasing light intensity (Speakman 1995; Russo et al. 2011a; Russo et al. 2011b; Chua and Aziz 2019; Vivas-Toro and Murillo-García 2020). There is no detailed information on the selection of prey during the day in most species of bats that exhibit these habits, however, P. pygmaeus tends to feed on insect swarms when available, particularly nematocerans flies, and on the most abundant taxa, such as brachycerans (Russo et al. 2011b).
Various studies have shown that bats foraging emergence times are closely related to canopy cover and forest closure (Russo et al. 2007; Marques et al. 2015), being common the occurrence of earlier emergencies in cluttered areas compared with open areas (Jones et al. 1995; Russo et al. 2007; Thomas and Jacobs 2013). This has been associated to strategies to evade the detection of predators or the risk of hyperthermia (Speakman 1995) and to maximize foraging time (Jones and Rydell 1994; Duvergé et al. 2000). Furthermore, the early emergence and late return of pregnant and lactating females in some species has been observed, which has been considered a strategy to feed for longer to meet energy demands during reproduction (Shiel and Fairley 1999; Duvergé et al. 2000; Lee and McCracken 2001).
There are currently 15 bat species reported for Gorgona Island, 9 of them are insectivorous (Murillo et al. 2014). Since S. leptura is abundant on Gorgona and exhibits an extended active period (Vivas-Toro and Murillo-García 2020), its insular population is suitable for studying diet composition and feeding habits of the species. Therefore, the aim of this study is to describe the diet composition of S. leptura, based on the daytime foraging of an insular resident population. Particularly, we seek to answer to the following questions: 1) are there differences in S. leptura prey consumption according to the canopy cover? 2) Are there differences in prey selection between males and females? 3) Is daytime feeding a good representation of the diet of S. leptura?
Materials and Methods
This study was conducted in Gorgona National Natural Park (GNNP; 2° 58’ 4’’ N, -78° 11’ 3’’ W), a continental island of volcanic origin located 35 km from the Pacific coast of Colombia (Giraldo 2012). The island has an equatorial climate with super-humid rainforest vegetation, that is part of the Chocó biogeographic province, and is mainly constituted by secondary forest (Vásquez-Vélez 2014). We determined S. leptura´s diet by analyzing stomach content and fecal samples collected in roosts only occupied by S. leptura, or from bats captured in flight. Sampling was carried out over 10 days between July and November 2017, five days during each month, in areas with high (≥ 70 %) and low (≤ 35 %) plant canopy cover. We collected fecal samples from roosts between 8:00 and 15:00 h. We collected fresh scats which were no more than four hours old; that is, of a soft consistency; a moist, dark color; and recognizable odor. We consider the foraging territory of bats in roosts, who was recorded in a previous study (Vivas-Toro and Murillo-García 2020), to define what type of canopy cover the samples belonged to. We captured bats between 17:00 and 19:00 h using a hand net and kept them in individual cloth bags until they defecated. All individuals were released after collecting the droppings. We carried out all captures and management of bats following the protocol for obtaining data on mammals on GNNP (Murillo et al. 2011). We stored the samples individually in plastic vials filled with 80 % alcohol. We also analyzed the stomach content of a recently dead individual (not collected), found around 11:00 h in November. Since in most cases several taxonomic groups were detected within a single scat, for a better standardization of the samples, and to avoid possible biases in the subsequent analyzes (according to Whitaker et al. (1996) for differences in size or volume of the samples), each scat (of approximately 6 x 2 mm) was considered as an independent unit. We identified prey at the most specific taxonomic level possible, in most cases to family, based on all recognizable fragments such as legs, wings, antennae, heads, tegument, scales, and/or structures with diagnostic characteristics (Whitaker et al. 2009). We estimated prey size by reconstructing them by grouping structures and fragments found using a Nikon SMZ745 stereomicroscope. All the material was identified, size estimated and quantified according to the taxonomic criteria and knowledge of an expert taxonomist.
We calculated the frequency of occurrence (FO) as the number of scats including the taxa divided by the total number of scats; the percentage composition (C) as the number of individuals of each taxon divided by the total number of individuals from all taxa, multiplied by 100 (Deagle et al. 2018; Vallejo et al. 2019); and the percentage volume (V) as the total volume of each taxon in the fecal-stomach samples divided by the total volume of all samples, multiplied by 100 for each taxon within the samples. We used the spheroid volume formula to approximate the volume value for each prey (Toshiaki 2004). We performed an arcsine transformation of all percentage data to correct non-normality before the analyses (Zar 1984). We used V to determine whether there were significant differences in diet between canopy covers and sexes through independent non parametrical ANOVAs, since the data was not normally distributed. Additionally, we tested differences in prey consumption by order relating the percentage volume of each order with each independent variable (canopy cover and sex) trough simple lineal regressions (Moosman et al. 2012) or fitted for quasi-Poisson distributions according to prior examination of the distribution and dispersion of each data set. All the analyses were conducted in R v.4.0.2 (R Development Core Team 2021).
Results
Intestinal content. We collected 63 fecal samples, 24 pellets from 7 bats captured in flight and 39 pellets from roosts; 29 pellets in low canopy cover and 34 in high canopy cover; 29 pellets were from males, 13 from females, and 21 mixed samples from a roost occupied by 2 males and 2 females. We identified 493 prey items belonging to 9 orders and 23 families of insects (Table 1). We also found mites (Arachnida), which are not intentionally ingested, but are present due to their phoretic relationship with the consumed insects; thus, they were not considered in the analyses.
The average size of prey eaten by S. leptura on Gorgona island was 5.1 ± 2.1 mm, ranging from 2 to 13 mm. The most abundant orders found were Hymenoptera (C = 32.25 %), Coleoptera (C = 30.83 %) and Hemiptera (C = 20.08 %; Figure 1), with Formicidae as the most consumed family (C = 18.46 %), followed by Chrysomelidae (C = 7.91 %) and Miridae (C = 7.71 %). The order Hymenoptera also comprised the highest percent volume (V = 36.25 %), followed by Hemiptera (V = 32.08 %) and Coleoptera (V = 21.18 %; Figure 1); with Formicidae as the most consumed family (V = 26.65 %), followed by Miridae (V = 15.46 %) and Chrysomelidae (V = 13.62 %) respectively. We identified the genus Trigona (stingless bees) and Camponotus (carpenter ants, two winged-morphospecies; Hymenoptera), representing new records in the diet of these bats. We also found two cephalic capsules of lepidopteran larvae.
Table 1 Diet of Saccopteryx leptura in Gorgona Island. Frequency of occurrence (FO), percentage composition (C) and percentage volume (V) of prey found in S. leptura feces. N = number of individuals.
| Order | Suborder/Family | Minimum taxa level | N | FO | C | V |
|---|---|---|---|---|---|---|
| Blattodea | 3 | 0.05 | 0.61 | 0.88 | ||
| Ectobiidae | 1 | 0.02 | 0.2 | 0.72 | ||
| Undetermined | 2 | 0.03 | 0.41 | 0.15 | ||
| Isoptera | Termitidae | 9 | 0.14 | 1.83 | 2.05 | |
| Hemiptera | 99 | 0.76 | 20.08 | 32.08 | ||
| Delphacidae | 3 | 0.06 | 0.61 | 0.97 | ||
| Cicadellidae | 24 | 0.29 | 4.87 | 4.66 | ||
| Reduviidae | 2 | 0.03 | 0.41 | 1.16 | ||
| Cixiidae | 1 | 0.02 | 0.2 | 0.18 | ||
| Rhyparochromidae | 3 | 0.05 | 0.61 | 0.37 | ||
| Miridae | 38 | 0.38 | 7.71 | 15.46 | ||
| Auchenorrhyncha | 6 | 0.11 | 1.22 | 1.27 | ||
| Fulgoromorpha | 6 | 0.08 | 1.22 | 2.25 | ||
| Undetermined | 16 | 0.25 | 3.25 | 6.18 | ||
| Psocoptera | 23 | 0.35 | 4.67 | 2.4 | ||
| Psocidae | 6 | 0.1 | 1.22 | 0.77 | ||
| Epipsocidae | 3 | 0.05 | 0.61 | 0.64 | ||
| Undetermined | 14 | 0.21 | 2.84 | 0.98 | ||
| Coleoptera | 151 | 0.86 | 30.83 | 21.18 | ||
| Chrysomelidae | 39 | 0.54 | 7.91 | 13.62 | ||
| Curculionidae | 30 | 0.37 | 6.09 | 4.47 | ||
| Scolytinae | 28 | 0.35 | 5.68 | 4.35 | ||
| Platypodinae | 2 | 0.03 | 0.41 | 0.12 | ||
| Endomychidae | 1 | 0.02 | 0.2 | 0.35 | ||
| Staphylinidae | 6 | 0.11 | 1.22 | 1.23 | ||
| Undetermined | 76 | 0.59 | 15.42 | 1.64 | ||
| Neuroptera | Undetermined | 1 | 0.02 | 0.2 | 0.55 | |
| Hymenoptera | 159 | 0.79 | 32.25 | 36.25 | ||
| Formicidae | 91 | 0.7 | 18.46 | 26.65 | ||
| Myrmicinae | 18 | 0.21 | 3.65 | 0.87 | ||
| Formicinae | 20 | 0.19 | 4.06 | 5.49 | ||
| Camponotus sp. 1 | 28 | 0.3 | 4.46 | 13.08 | ||
| Camponotus sp. 2 | 14 | 0.19 | 2.84 | 5.47 | ||
| Undetermined | 17 | 0.19 | 3.41 | 1.75 | ||
| Apocrita (parasitica) | 1 | 0.02 | 0.2 | 0.01 | ||
| Braconidae | 16 | 0.17 | 3.25 | 1.26 | ||
| Ichneumonidae | 2 | 0.03 | 0.41 | 0.32 | ||
| Halictidae | 30 | 0.38 | 6.09 | 4.66 | ||
| Apoidea | 1 | 0.03 | 0.2 | 0.26 | ||
| Apidae | 15 | 0.17 | 3.04 | 2.49 | ||
| Trigona | 10 | 0.11 | 2.03 | 1.04 | ||
| Undetermined | 5 | 0.08 | 1.01 | 1.45 | ||
| Vespoidea | 2 | 0.02 | 0.41 | 0.34 | ||
| Lepidoptera | 8 | 0.08 | 1.62 | 0.8 | ||
| Noctuidae | 4 | 0.03 | 0.81 | 0.37 | ||
| Undetermined1 | 2 | 0.02 | 0.41 | 0.06 | ||
| Undetermined | 2 | 0.03 | 0.41 | 0.25 | ||
| Diptera | 36 | 0.44 | 7.3 | 3.81 | ||
| Muscomorpha | 2 | 0.03 | 0.41 | 0.3 | ||
| Nematocera | 3 | 0.05 | 0.61 | 0.21 | ||
| Ulidiidae | 1 | 0.02 | 0.2 | 0.12 | ||
| Dolichopodidae | 1 | 0.02 | 0.2 | 0.62 | ||
| Sciaridae | 1 | 0.02 | 0.2 | 0.02 | ||
| Phoridae | 1 | 0.02 | 0.2 | 0.06 | ||
| Undetermined | 27 | 0.37 | 5.48 | 2.47 | ||
| Undetermined | 3 | 0.03 | 0.61 | - |
1 Undetermined Lepidoptera family represented by larvae cephalic capsules.
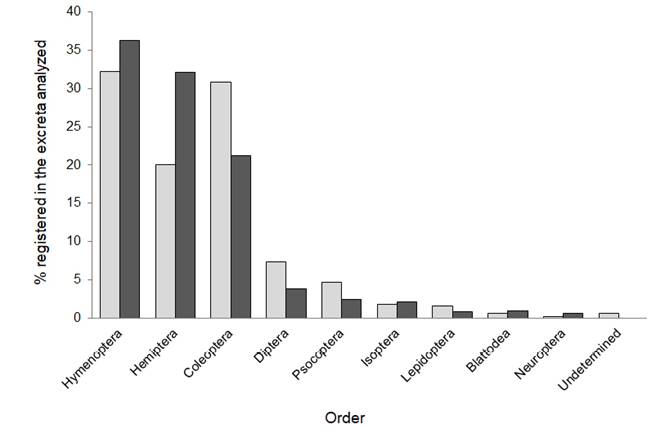
Figure 1 Percentage composition (light gray) and percentage volume (dark gray) of insect orders consumed by Saccopteryx leptura on Gorgona Island.
We found no significant differences in the diet of S. leptura between between plant canopy covers (X 2 = 0.004, df = 1, P > 0.05) or between sexes (X 2 = 0.370, df = 1, P > 0.05). When analyzing by order, we only found significant differences for Psocoptera, which were consumed more often in low canopy cover (F 1,61 = 9.38, P < 0.01; Figure 2a) and by males (F 1,40 = 6.62, P < 0.05; Figure 2b). That is, a volume of 74.40 % in low canopy cover vs. 25.60 % in high cover, and 90.27 % in males vs. 9.73 % in females from the total volume of Psocoptera consumed.
Stomach content. The stomach content of the S. leptura male we found dead during the study consisted of insects almost entirely of the order Hymenoptera (C = 94.40 %), with remains from the families Braconidae and Formicidae, and the subfamilies Myrmicinae and Formicinae (Camponotus sp.1 and Camponotus sp.2; Table 2); and a buprestid beetle (C = 5.60 %). The largest prey found was a 13 mm Camponotus sp.2 ant; while the smallest was a 3 mm braconid wasp.

Figure 2 Variation in the diet of Saccopteryx leptura in Gorgona island in relation to (A) canopy cover and (B) sex. Percent volume and standard error are illustrated for each insect order. Asterisks represent significant differences in the diet of S. leptura according to individual regression tests for each order (* = P < 0.05; ** = P < 0.01). Colors code: orange, low canopy cover; green, high canopy cover; blue, males; red, females.
Discussion
Insectivorous bats tend to be selective with their food, their preferences may depend on features such as hunting methods and echolocation, prey characteristics such as size and texture (Barclay and Brigham 1994; Jung et al. 2007; Sedlock et al. 2014), the bat’s cranial morphology, among others (Freeman 1979; Van Cakenberghe et al. 2002). Also, several studies have demonstrated that they can be flexible in terms of food selectivity according to the availability and seasonality of their prey (Whitaker 1994; Whitaker et al. 1996; Agosta et al. 2003), by inter or intraspecific competition (Whitaker 2004; Novella‐Fernandez et al. 2020) and even due to their reproductive and/or developmental states (Verts et al. 1999; Agosta and Morton 2003). According to the stomach and fecal samples content found in this study, the insular population of S. leptura on Gorgona had a considerable variety of prey in its diet compared with what was previously reported in continental populations (Nogueira et al. 2002; Cruz-Parrado et al. 2018), and the approximations for S. leptura populations in Trinidad Island (Bradbury and Vehrencamp 1976). In general, we found that Saccopteryx leptura feeds on a wide variety of prey, from soft insects such as Psocoptera, Diptera and Lepidoptera; to harder insects such as Coleoptera and Hemiptera. For example, the relative consumption of Hymenoptera, in terms of composition and volume, was mainly determined by the FO of winged formicids in the samples (70 %). The latter can be explained by the abundance of these insects in the island since they are social insects whose populations and colonies are very numerous (Universidad del Valle 2014). Additionally, the caste of reproductive (winged) individuals within their colonies perform daytime nuptial flights consisting of hundreds of individuals, (Quirán and Corró 1998; Lara-Juárez et al. 2015). The consumption of winged ants has been previously reported in S. leptura´s diet, representing one of its most frequent prey (Nogueira et al. 2002; Cruz-Parrado et al. 2018). Many tropical bats feed on seasonal nuptial swarms of termites or ants, since they involve an important food resource, clustered in one single area (Kunz et al. 1995; Pavey et al. 2001). There are even species such as Rhinopoma microphyllum that can feed almost exclusively on Camponotus alates during their summer massive nuptial flights (Levin et al. 2009). Therefore, since S. leptura also feeds during the day, it makes perfect sense that it takes advantage of the activity patterns of these formicidae swarms (mainly Camponotus) as an abundant food resource to complement its diet. It is worth noting that, as far as we know, the only prey identified at genus level in S. leptura´s diet is Pheidole (big-headed winged ant; Nogueira et al. 2002), therefore, both Camponotus and Trigona (Apidae) represent new records within the diet of these bats.
Similarly, the high consumption of beetles may be because Coleoptera is a very abundant order of flying insects on the island (Universidad del Valle 2014), and very accessible to the bats even during the daytime, which is demonstrated by the highest FO (86 %) among the insect orders in the samples. This coincides with the study conducted by Bradbury and Vehrencamp (1976), which demonstrated that the foraging cycles of S. leptura are spatially and temporally correlated with patterns of insect abundance in riparian forests in Costa Rica and Trinidad Island. On the latter, daytime flight was also reported for the species under the canopy. The average size of prey eaten by these bats in Gorgona (5.1 ± 2.1 mm) was almost twice the expected size of 2.6 mm suggested for bats of similar sizes (Bradbury and Vehrencamp 1976). Also, the smallest prey captured (2 mm) was 1.3 times smaller than the expected average suggested by Bradbury and Vehrencamp (1976), and the largest (13 mm), was 2.6 times larger than that reported by Nogueira et al. (2002). Therefore, our results demonstrate the plasticity of S. leptura prey selection, compared with many insectivorous species that are very strict with their prey selection (Burles et al. 2008; Kolkert et al. 2019). This could be considered a strategy of the species to take advantage of as many resources as possible on the island through opportunism. Opportunistic feeding behavior has been recorded in many bats under a variety of environmental and competitive pressures (Brigham et al. 1992; Heim et al. 2017), showing a tendency to consume locally abundant taxa (Whitaker 2004; Krauel et al. 2018). Note that the representativity of other soft insects, such as Diptera, which is a very abundant group on Gorgona, and which have sizes within the range of prey consumed by S. leptura, could be underestimated, since they are easily destroyed during digestion. It is also worth noting the presence of two cephalic capsules of lepidoptera larvae within the food remains as an atypical finding, since, from what is known, S. leptura only hunt airborne prey; gleaning or trawling behaviors have not been reported to date (Kalko 1995; Jakobsen et al. 2015). However, this could be explained by the fact that there are larvae of Noctuidae and Geometridae (both present in the island) that, in their first development stages, hang on silk threads to evade parasitoids and predators (Hagstrum and Subramanyam 2010), thus, facilitating its detection and predation by S. leptura.
The significant differences in prey consumption, which were only found in order Psocoptera, can be attributed to the distribution of these insects in different microhabitats of Gorgona, since they are more abundant in low canopy coverage areas (Sarria et al. 2014), and because in tropical moist forests they are usually found in the middle to upper part of the vertical strata, which just overlaps with the daytime feeding territory of these bats. Saccopteryx leptura usually chooses specific feeding territories that they defend constantly (even for generations), below the canopy during the day, and in open areas above the canopy at night (Bradbury and Emmons 1974; Bradbury and Vehrencamp 1976; Kalko 1995). Something similar could be happening between males and females, since we observed that it is common for individuals that roost together to share their feeding territories both simultaneously and asynchronously. If we consider the assumption that S. leptura feeds on the insects available in its territory, then the chances of differentiation in prey consumed between the sexes would decrease considerably. Thus, it is possible that the heterogeneity in the diet of this species is mostly determined by the richness, composition, and abundance of prey in its hunting areas as well as its ability to locate and capture them, rather than some type of prey preference or specialization.
Table 2 Percentage composition (C) and percentage volume (V) of prey found in the stomach content of a dead male specimen of Saccopteryx leptura found in Gorgona island. N = number of individuals.
| Order | Family | Subfamily | Minimum taxa level | N | C | V |
|---|---|---|---|---|---|---|
| Coleoptera | 1 | 5.6 | 0.4 | |||
| Buprestidae | 1 | 5.6 | 0.4 | |||
| Hymenoptera | 17 | 94.4 | 99.6 | |||
| Formicidae | Myrmicinae | 7 | 38.9 | 3.8 | ||
| Formicinae | Camponotus sp. 1 | 4 | 22.2 | 49.6 | ||
| Camponotus sp. 2 | 5 | 27.8 | 45.1 | |||
| Braconidae | 1 | 5.6 | 1.2 |
Although we only limited our sampling to S. leptura daytime activity, we consider our data to be a valid approach to trophic habits of the species. However, considering the type of food consumed and the activity of some insects found in our samples, we can infer that their diet composition during the day and during the night is not completely homogenous. For example, the presence of bees such as Trigona sp., winged ants and diurnal chrysomelid beetles in the samples analyzed are irrefutable proof that there is some diet differentiation with respect to their conspecifics and other nocturnal insectivorous bats species residing on the island. Although there is no previous research in insectivorous bats with diurnal habits that explore the differences between the diurnal and nocturnal diet, differentiation has been previously suggested (Russo et al. 2011a). However, when comparing the results of diet surveys for Pipistrellus pygmaeus during the night (Bartonička et al. 2008) and the day (Russo et al. 2011b) to provide an example, we did not observe noticeable differences in the main food resources, but in insects whose frequency of occurrence seems to be related to the bats’ foraging activity and the characteristics of the foraging sites. Thus, more detailed studies focused on this topic are required for a better understanding of the general diet of these species. In the same way, more studies providing a better understanding of the night-time prey selection S. leptura are still required.
Our data is sufficient to suggest that the population of S. leptura in GNNP has a heterogenous and opportunistic diet, without an apparent preference for a particular type of insect, presumably to take advantage of the most abundant and available seasonal resources. It is important to increase the research on the prey selection of this species on the mainland in order to determine whether the diet composition of S. leptura on the island is locally exclusive or is generalized for the species. Studying insular species allows us to inquire about the strategies and adjustments that their populations use to survive, given the resources that the environment provides. Thus, studying the diet of other resident species would increase our understanding of the similarities and differences between them, and would allow us to determine how these species contribute to the balance of the ecosystem on the island.











 nueva página del texto (beta)
nueva página del texto (beta)


