Introduction
White-tailed deer (Odocoileus virginianus) has the widest distribution and is the most abundant of all New World cervids (Teer 1994). Its distribution extends from southeastern Alaska in North America to northern South America with up to 38 recognized subspecies (Mattioli 2011). There is controversy regarding whether there is only one species or several species (Molinari 2007). In the latter case, the species found in Central America corresponds to Odocoileus cariacou of wide distribution on the Neotropical region (Molinari 2007).
Endogenous recognition of photoperiod changes drives seasonal reproductive cycles (Bronson 2009). A decrease in the daylight-darkness ratio triggers the onset of the reproductive cycle. Since the photoperiod is related to latitude (Ditchkoff 2011), the rutting season of white-tailed deer progresses along a continuum from November in most of North America, to January to March in northern México (Mattioli 2011), and from January to April in central and southern México (Weber et al. 1994; Contreras-Moreno et al. 2019). The annual cycle of antlers is related to the reproductive cycle with variation in the reproductive chronology linked to environmental variables mainly associated with latitude, especially photoperiods and food availability (Goss 1983; Price et al. 2005a; Hanon et al. 2008; Contreras-Moreno et al. 2019).
The annual phases of primary development of the testes, the complete production of sperm, and the rest period (Robinson et al. 1965) are associated with the onset of antler growth, shed of velvet, and antlers fall off, respectively (Verme and Ullrey 1984). The photoperiod acts through melatonin to modulate the secretion of reproductive hormones, mainly testosterone (Goldman 2001; Hanon et al. 2008) and regulates antlers cycle in white-tailed deer (Price et al. 2005a; Price et al. 2005b). However, because there is only a change in photoperiod in the tropics with a weak temporal association with seasonal climatic changes, it results that this environmental factor could have a reduced influence in controlling antler development in Southern México (Contreras-Moreno et al. 2019).
In temperate and cold regions of North America, severe weather and food availability are the critical environmental factors that dictate the timing of the birth season (Lincoln 1992; Price et al. 2005b). The reproductive cycle of white-tailed deer in these regions of North America is seasonal (Verme and Ullrey 1984; Mattioli 2011). The status of the antlers is associated with the growth and secretion cycle of testosterone by the testicles (Vasantha 2016). The testosterone cycle is governed largely by photoperiod (Loudon and Curlewis 1988; Tomás 1995). The decrease of daylight and the increase in testosterone cause antlers to mineralize and shed their velvet (Tomás 1995). In contrast, increased daylight and decreased testosterone cause antler casting (Vasantha 2016). On the opposite, there is little information about the reproductive aspects of white-tailed deer in the Neotropics (Contreras-Moreno et al. 2019). The birth of fawns and the development and casting of male antlers attracted the interest of the first naturalists working in the region (Rodríguez and Solís 1994). They speculated that fawns are born throughout the year and that there is no annual cycle in the growth and shedding of antlers due to the lack of seasonality in tropical and subtropical regions (Rodríguez and Solís 1994). However, studies conducted in the 1980s concluded that there may be some degree of synchrony in the growth of antlers and the birth of fawns in some places (Klein 1982; Branan and Marchinton 1987; Rodríguez and Solís 1994). Nonetheless, even in sites located nearby, the results seem contradictory because births occur in any season and the time of the year in which males shed their antlers is a function of individual age (Webb and Nellis 1981; Brokx 1984; Rodríguez 1994; Fuller et al. 2020).
Studies on the reproductive cycle of white-tailed deer in Costa Rica have yielded contrasting results with clearly seasonal cycles at San Lucas Island (Rodríguez and Solís 1994) and continuous cycles with two birth peaks in Palo Verde and Santa Rosa national parks (Rodríguez 1994; Fuller et al. 2020). In the Nicoya Peninsula in northwestern Costa Rica, a peninsular site south of these areas and a different life zone, we observed males with hard antlers in 2015 but only from July to November. As a result, we made extensive, regular field observations from 2016 to 2019 to analyze the antler cycle in a white-tailed deer population in this area. We also recorded fawn birth periods in these four years. We hypothesize that white-tailed deer have a continuous reproductive cycle during the year in Curú because the rainfall pattern in this area is the same as in Palo Verde and Santa Rosa National Parks, where this species has an almost continuous annual reproductive pattern.
Material and methods
Study site. The Hacienda y Refugio de Vida Silvestre Curú (Curú) combines private and public lands totalizing 1,496 ha: 312 ha dedicated to cattle ranching and 1,184 ha dedicated to conservation and ecotourism (Schutt and Vaughan 1995). It is located on the Nicoya Peninsula in northwestern Costa Rica (9° 47’ 23” N, -84° 55’ 28” W; Figure 1). Curú vegetation belongs to the Tropical Humid Forest Life Zone (Holdridge 1967; Bolaños et al. 2005).
Although the refuge is relatively small it is part of a continuum mosaic of cattle ranching operations and forest remnants along most of the south of the peninsula. Adjacent to Curú there is a secondary forest of over 5,000 ha located at the Peninsula de Nicoya Protected Zone. Curú is known to contain a high level of biodiversity due to the presence of several habitat types and its protection status (Schutt and Vaughan 1995). Seventy-eight species of mammals, 232 species of birds, 87 species of reptiles, and at least 500 species of plants have been identified in the refuge (McKinney 2014). Annual precipitation averaged 1,957 mm for the period 1970-2005, but it was 2,177 mm during our study (IMN 2020a; IMN 2020b).
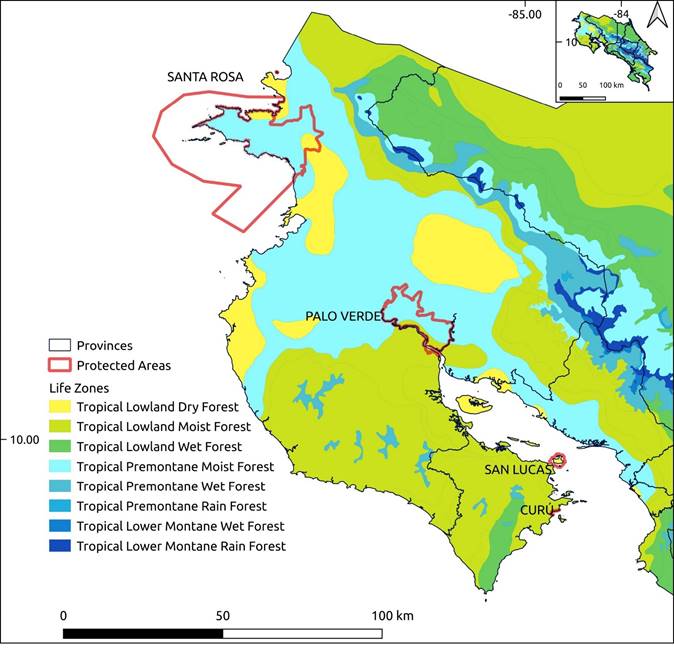
Figure 1 Life zones in Guanacaste province at Northwestern Costa Rica, and the four study sites mentioned in the text. The Hacienda y Refugio de Vida Silvestre Curú is located at the extreme southeastern portion of the Nicoya Peninsula in the Tropical Lowland Moist Forest. Map by G. Chaves (Cachí).
The dry season occurs from December to March when only 4 % of total annual rainfall accumulates. The transition to the rainy season is in April and by the third week in May, the rainy season is typically well established (IMN 2020). The first rainy period occurs between May and August when 50 % of the annual total precipitation accumulates. The first maximum of rains occurs in June as the southeastern trade winds intensify causing local storms and heavy downpours. The northeast trade winds intensify again causing a brief dry season between July and August called the “Pacific summer” or “canicula”. A second rainy season extends from September to November. The greatest amount of rain, 46 % of the annual total, falls during September and October due to the greater influence of Caribbean cyclonic events. The transition to the dry season occurs in November (IMN 2020).
Data collection. We visited Curú once a month between January 2016 and December 2019, except in September 2016 and September 2019 when it was impossible to enter the area due to flooding and landslides on the access road. However, this did not affect our study since September is not of high significance for our purposes. We looked for deer on two transects: the main road (2,090 m) and a secondary road (1,958 m). We walked each transect in the morning (6:00 h to 9:00h) and the afternoon (15:00h to 17:00h) every month. We recorded deer data using 10 x 50 binoculars and a digital camera.
Every time we observed a deer we recorded the sex and age group (fawn, yearling, adult) of each individual. We classified adult males by the presence of antlers and testicles and adult females by the absence of antlers and testicles, as well as by the presence of one or two fawns. Juvenile deer were classified as males or females based on body size and by the presence or absence of pedicels and testicles, respectively. Fawns were identified by the presence of spots on their fur. Both researchers have vast experience identifying and classifying deer by age.
Each time we observed an adult male we took a digital photograph for a database on the monthly growth status of deer antlers. For individual recognition of males and to avoid double-counting, we considered: 1) antlers size, 2) antler shape (number of tips, malformations) and 3) physical characteristics of each animal (presence of scars and wounds). Later we classified adult males as 1) nubs (no antlers or just one antler, pedicels in velvet), 2) velvet (growing antlers covered in velvet with rounded tine ends), and 3) hard (fully formed antlers, velvet-less; Figure 2). Additionally, we recorded rubs, odor marks, nonaggressive sparring, fights matching dominant bucks, chasing, and tending bucks, and bucks scraping the ground and leaving odor marks locally known as “rascaderos” (Rodríguez 2015). Although we also recorded the presence of adult females in the field, we made no effort to identify them individually. At each visit, we also counted spotted fawns and recorded them as small and large spotted fawns (see below).
Data interpretation and assumptions. All data were analyzed by month and year, however, for this report, the data were grouped for the whole study period: 2016 to 2019. To identify the reproductive seasonality of deer, we made the following assumptions concerning deer morphology and development based on a literature review. Antlers are shed annually (Sauer 1984). Antler’s growth lasts six months, and velvet dries and falls. Adult males keep hard antlers for about six months and then shed them (Sauer 1984). Testis’s cycle has three phases: 1) primary development, 2) full sperm production, and 3) resting period (Verme and Ullrey 1984). These phases are associated with the onset of antler growth, velvet shedding, and antler casting respectively (Verme and Ullrey 1984). Adult males are fertile only during the hard antler period. Female gestation varies among subspecies and ranges from 187 to 222 days, with a mean of 202 days (Smith 1991). Fawns spend most of their time hidden away from their mothers for three to four weeks (Marchinton and Hirth 1984), but gradually fawns spend more time with their mothers.
It is not possible to know the date of birth of the fawns in the field. Therefore, we classified spotted fawns into two groups: 1) small spotted fawns were two weeks to two months old, they do not reach the height of the mother’s belly, and they are generally observed alone in the forest or accompanied by the mother, and 2) large spotted fawns were three to four months old, they exceed the height of the mother’s belly, and they are usually accompanied by the mother and other deer.
To test for the independence between the status of antler growth and the month of the year (which are related to the amount of rain) we analyzed the data with a Chi Square Test. We compared the absolute number of white-tailed deer (1,134) according to antler status per month. We used the adjusted residuals of the Chi Square Test as a posteriori test to evaluate in which cells of the contingency table it is observed more or less frequently than is expected at random. The critical value is the range from -1.96 to 1.96, so the values outside this range differ from the frequency expected by pure chance (Table 2).
Results
We totalized 1,134 observations of the status of antler growth of male white-tailed deer at Curú (n = 233 in 2016, n = 367 in 2017, n = 294 in 2018 and n = 240 in 2019; Table 1). The way we walked the transects and our knowledge of the area reduced the probability to count any male more than once. Additionally, we recorded 13 observations on reproductive behavior and 133 observations of spotted fawns.
Antlers. The antler cycle of the white-tailed deer at Curú was seasonal in the four years of study (χ2 = 970, d.f. = 22, P < 0.001). Antlers casting occurred over a three-month period that began in mid-November and ended in mid-February, during the first part of the dry season. The earliest antlerless or single-antlered deer were observed on 19 November 2017. The last single-antlered deer was observed on 5 February 2017. Antlerless or single-antlered males were not observed in any other month. Antlers grew during the dry season from mid-December to mid-March. A hundred percent of the males had velvet antlers in March in the four years of study (Figure 3a, Table 1).
Table 1 The absolute number of white-tailed deer (Odocoileus virginianus) according to antler status per month in the Hacienda y Refugio de Vida Silvestre Curú between January 2016 and December 2019. Nicoya Peninsula, Costa Rica.
| Antler status | ||||
|---|---|---|---|---|
| Month | Hard | Velvet | Nubs | n |
| Jan | 33 | 37 | 47 | 117 |
| Feb | 4 | 106 | 9 | 119 |
| Mar | 0 | 96 | 0 | 96 |
| Apr | 7 | 93 | 0 | 100 |
| May | 25 | 78 | 0 | 103 |
| Jun | 75 | 42 | 0 | 117 |
| Jul | 88 | 2 | 0 | 90 |
| Aug | 82 | 0 | 0 | 82 |
| Set | 42 | 0 | 0 | 42 |
| Oct | 70 | 0 | 1 | 71 |
| Nov | 69 | 1 | 8 | 78 |
| Dec | 80 | 15 | 24 | 119 |
| Total | 1,134 |
x2= 970, d.f. = 22, P < 0.001.

Figure 2 Antler growth stages of white-tailed deer (Odocoileus virginianus) at Hacienda y Refugio de Vida Silvestre Curú, Northwestern Costa Rica: a) nubs, b) velvet, c) hard.
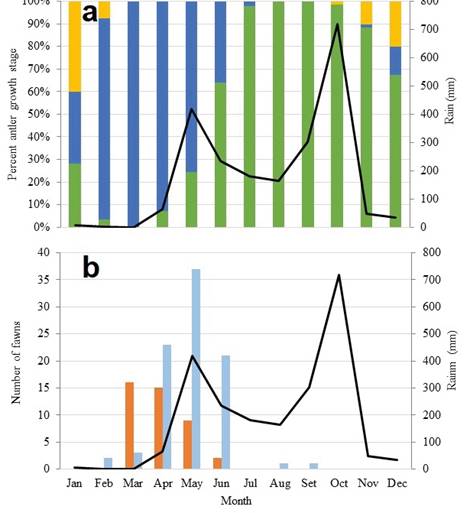
Figure 3 a) Monthly relative frequency (%) of antler status (n = 1134, monthly range 42-119). Hard (green), velvet (blue) and nubs (yellow). b) number of monthly observations of small spotted fawns (orange) and large spotted fawns (light blue) of white-tailed deer (Odocoileus virginianus) in Hacienda y Refugio de Vida Silvestre Curú between January 2016 and December 2019. Black lines indicate the average monthly precipitation (mm) in the same period at the Paquera Station: 2016 = 1,473.4 mm; 2017 = 2,475.8 mm; 2018 = 2,436.4 mm; 2019 = 2,327.8 mm (IMN 2020a), Nicoya Peninsula, Costa Rica.
Table 2 Value of adjusted residuals of the χ2 test for antler status of white-tailed deer (Odocoileus virginianus) per month in the Hacienda y Refugio de Vida Silvestre Curú Nicoya Peninsula, Costa Rica, between January 2016 and December 2019. Residuals in bold indicate significant differences. The value of the sign indicates if the frequency is higher (+) or lower (-) than expected by chance.
| Antler status | |||
|---|---|---|---|
| Month | Hard | Velvet | Nubs |
| Jan | -5.14 | -2.277 | 13.728 |
| Feb | -10.919 | 11.148 | -0.122 |
| Mar | -10.387 | 12.172 | -2.988 |
| Apr | -9.155 | 10.959 | -3.056 |
| May | -5.628 | 7.407 | -3.106 |
| Jun | 3.061 | -1.286 | -3.333 |
| Jul | 9.309 | -7.872 | -2.885 |
| Aug | 9.27 | -7.909 | -2.744 |
| Set | 6.512 | -5.556 | -1.927 |
| Oct | 8.336 | -7.322 | -2.084 |
| Nov | 6.912 | -7.462 | 0.819 |
| Dec | 3.81 | -6.75 | 5.282 |
Velvet shedding started in April and lasted through May and June, just at the beginning of the rainy season. In July less than 5 % of the males presented velvet antlers. We did not observe males with velvet antlers from August to October. We observed males with hard antlers from April until January or February of the following year. From July to October, 100 % of the males had hard antlers (Figure 3a, Table 1).
There was a significantly lower frequency of hard antlers than expected by chance in the first five months of the year, and significantly more frequency from June to December (Table 2). On the opposite, there was significantly higher frequency of antlers in velvet than expected from February to May and a lower frequency from July to January of the following year. Nubs were less frequent than expected in general during the year except in January and December, although differences were not significant in February, September, and November (Table 2).
Rutting season. The rutting season of white-tailed deer in Curú Refuge lasted three months from mid-June to mid-August. Rutting began when adult males were ready to reproduce, indicated by their hard antlers and reproductive behavior. In mid-June, males started rubbing antlers, scraping, and sparring. This behavior lasted for the first part of the rainy season. They adopted tending bond mating systems, courtship and copulating receptive does in August and September when males had hard antlers (Figures 3a, 4). The first males observed chasing does entering estrus were in August. We observed does accompanied by bucks in August and September, and by October neither chasing nor courtships were observed (Figure 4).
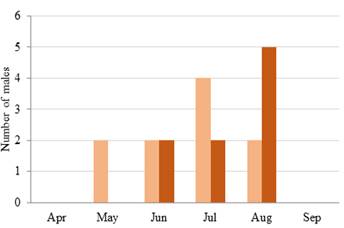
Figure 4 The number of males white-tailed deer (Odocoileus virginianus) per month that chased females, and the number of males per month that accompanied females, between January 2016 and December 2019 (other months have cero males engage in these activities). Hacienda y Refugio de Vida Silvestre Curú, Nicoya Peninsula, Costa Rica. Chasing deer males (pink) and tending deer males (brown).
Fawning season. Small spotted fawns (Figure 5) were observed between March and June (Figure 3a); this is from the middle of the dry season to the beginning of the rainy season. During March most of the observations were of small spotted fawns (16 out of 19), while only three observations corresponded to large spotted fawns (Figure 3a). On the opposite, observations of large spotted fawns (Figure 5) predominated in April (23 out of 38).
A peak of spotted fawns was reached in May, although most of them were large-bodied spotted fawns (37 out of 46). Large-bodied spotted fawns (21 out of 23) also predominated in June when only two small spotted fawns were observed (Figure 3a). Fawns were five months old by July and had no spots. We did not see any spotted fawns after June, except by two large spotted fawns in August and September 2017, that were born probably by April-May given their sizes.
Discussion
Because there are comparatively few studies of white-tailed deer in the Neotropical region, a general conclusion emerged over time that white-tailed deer would be less seasonal in antler growth and casting in regions without strong seasonal changes in weather (Marchinton and Hirth 1984). The assumption was that the same would happen with other reproductive processes such as rut and fawn births. However, some studies challenge this generalization. White-tailed deer reproduction seemed seasonal, lasting four to six months, as reported from limited observations in the Virgin Islands (Webb and Nellis 1981), Honduras (Klein 1982), Venezuela (Brokx 1984), Colombia (Blouch 1987) and Surinam (Branan and Marchinton 1987). A recent study in Campeche wetlands in Southeastern México, a tropical site, showed that the antler cycle of the white-tailed deer is seasonal (Contreras-Moreno et al. 2019).

Figure 5 a) Small spotted fawn and b) large spotted fawn of white-tailed deer (Odocoileus virginianus) at Hacienda y Refugio de Vida Silvestre Curú, Northwestern Costa Rica.
Studies of white-tailed deer reproduction in Costa Rica have shown considerable variation in reproductive processes among relatively nearby populations. Rodríguez (1994) found no seasonality in antlers casting and growth or in fawn births in Palo Verde National Park in the mid- 1990s and recently Fuller et al. (2020) obtained similar results in a study in Santa Rosa National Park. However, on San Lucas Island, situated approximately 78 km in a straight line from Santa Rosa, the white-tailed deer life cycle had a marked seasonality (Rodríguez and Solís 1994). Antler growth occurred from March to June, velvet shedding in June and July, hard antlers from July to December, and antler casting in January and February (Rodríguez and Solís 1994). Antler status pattern, as well as fawn births, were even more seasonal in Curú than in San Lucas Island, the only real dry forest in Costa Rica included in this comparison (Figure 1). These processes took three months to complete in Curú. Some minor variations in the onset of antler casting and velvet shedding appear to be responses to variations in precipitation patterns between consecutive years. The first signs of the start of the rut appeared in June in Curú when antler velvet had dried and shed, and bucks had hard antlers (Figure 2c). Antler cycle as well all other processes were significantly seasonal (Table 2). Antler cycle seasonality in Curú coincides with the antler cycle of the white-tailed deer in a tropical site in southeastern México (Contreras-Moreno et al. 2019). In Curú, fawns were five months old by July and had no spots, which means they were born in February but were not observed sooner due to their hiding behavior during the first weeks of life (Rodríguez 2015).
Rain seasonality at Santa Rosa National Park (Fuller et al. 2020) is similar to that of Curú. Rainfall peaks in May - June, decrease in July - August, and peaks again with greater precipitation around October. That could explain why the phenology of Curú vegetation demonstrates similar patterns to those of other sectors in Northwestern Costa Rica where many dry forest species are deciduous and recover much of their foliage at the beginning of the rainy season in May and June (Castro et al. 2018). However, several tree species maintain foliage throughout the dry season, as do half of the shrubs, saplings, and seedlings (Frankie et al. 1974; Opler et al. 1980), and many animal species reflect these patterns (Rockwood 1975; Janzen 1993; Orkin et al. 2019).
Curú is located in a more humid life zone than Santa Rosa (Figure 1), but this seems to not affect the white-tailed deer reproductive cycle. Although the rainy season determines the availability of food, which is a proximate cause of high variation of the reproductive cycle in Santa Rosa during the year (Fuller et al. 2020), it does not seem to have a strong influence on the deer cycle in Curú. However, there was a coincidence between the peak of precipitation occurring in May and the highest number of fawns with spots (36, Figure 3a).
Lack of seasonal variation in the photoperiod is the decisive factor that likely favor deer reproduction throughout the year in Santa Rosa (Fuller et al. 2020).Yet this variation is identical in Curú, where the reproduction of the species is seasonal. The peak of births in February and March ensures the survival of the fawns, which will be weaned at the beginning of the rainy season when there is plenty of food. The high amount of rain in September and October, including flooding, will not affect the fawns because they are already grown up by this time of the year (Figure 3a). However, these severe rains and flooding could be an impacting seasonal factor on the fawning season at Curú. The association of flooding with changes in the fawning season has been reported in white-tailed deer populations that occur in areas affected by severe flooding such as the Everglades in Florida (MacDonald-Beyers and Labisky 2005).
The reproduction pattern we observed in Curú is similar to the pattern on San Lucas Island, located in a dry forest at a distance less than 20 km in a straight line (Rodríguez and Solís 1984). What is different among the sites? The traditional or more commonly used system of ecosystem classification in Costa Rica is Holdridge´s Life Zones (Bolaños et al. 2005). In this classification, Santa Rosa and Palo Verde are in the Pre-montane humid Forest Life Zone and Curú in the Tropical humid Forest zone, leaving San Lucas Island as the only one of the sites included in our comparison that is located in a tropical dry forest. This explains to some degree the highly seasonal reproductive process of the white-tailed deer in San Lucas, but it does not explain the seasonality pattern we found in Curú. However, Curú is even more humid than Santa Rosa and Palo Verde, even though all three of these sites as well as San Lucas have a marked dry season. Rainfall in Curú is higher than in Santa Rosa, and it may make a difference in year-round food availability, but severe rains and flooding by September and October can have a more determining impact on fawning seasonality.
The reproductive patterns of white-tailed deer in Central and South America may have evolved in response to seasonal fluctuations in specific food availability, competition, or predation, all of which may be related to rainfall patterns (Asher 2011). However, these factors do not explain why white-tailed deer reproduction is seasonal in some places, but not in others that are very close. Is there a direct relationship to weather or is some other factor that differs between the sites responsible for the differences in white-tailed deer reproduction? Are white-tailed deer more genetically related to North American forms that are more seasonal, or does another factor make them different from non-seasonal deer? It appears that white-tailed deer exhibit a very flexible and therefore variable reproductive pattern (Fuller et al. 2020). More research is needed to understand what factors more directly explain the observed pattern in the white-tailed deer reproduction.











 nova página do texto(beta)
nova página do texto(beta)


