Introduction
Rodents influence the processes of tropical forest regeneration and the structure and function of plant communities, since they prey on and disperse seeds (Sánchez-Cordero and Martínez-Gallardo 1998; Shiels and Drake 2011; Fleury et al. 2014), as well as consuming other plant parts (Meyer and Butaud 2009; Shiels et al. 2013). Likewise, they influence the populations of other groups through their role as predators of invertebrates and small vertebrates (Trujano-Alvarez and Álvarez-Castañeda 2010; Witmer and Pitt 2012). They use a wide range of ecological niches, and changes in their abundance and diversity reflect modifications in their habitat (Cimé-Pool et al. 2010; Whitehead et al. 2014).
In Mexico, few studies have described the biology, structure and population size of the small tropical rodents, especially those of tree-dwelling and semi-tree-dwelling habits (Schnell et al. 2010; Hernández-Betancourt et al. 2008, 2012; Panti-May et al. 2014). Equally, patterns relating to the use or selection of habitat have rarely been described in this group (Domínguez-Castellanos et al. 2007; Poindexter et al. 2013). For some rodents, this differential site selection depends on the structural and vegetal characteristics that comprise their specific microhabitat and that, in turn, affect their distribution and behavior. For example, Heteromys irroratus is associated with high proportions of dead plant material and most heteromids are generally recorded in certain combinations of stony sites with a cover typical of scrublands (Tapia-Ramírez et al. 2012). Likewise, microhabitat use can vary considerably according to the habitat structure, species population density and the inter-specific interactions (Bellows et al. 2001; Rojas-Martínez et al. 2012; Villanueva-Hernández et al. 2017). In this sense, it has been reported that the females of Peromyscus leucopus, unlike the males, present an association with different microhabitats that include the canopy, since they make greater use of the vertical space of the habitat in the search for resources, or as a response to competition for food due to high population density (Klein and Cameron 2012). Studies addressing the feeding habits of small rodents are scarce; however, these mammals play an important ecological role in the ecosystems they inhabit (Montenegro-Díaz et al. 1991; San-José et al. 2014). Some studies of small rodents have used methods of analysis of feces and stomach contents, since these techniques can provide reliable information regarding the material ingested by the species (e. g.,López-Cortés et al. 2007; Peralta 2015; Panti-May et al. 2019).
The group of small rodents includes the genus Peromyscus, which is the most diverse in Mexico. Some of its species have been studied from an ecology perspective (Morris et al. 2011; Dallas et al. 2012; Kalkvik et al. 2012), behavior (Weber and Hoekstra 2009; Williams et al. 2013), physiology (Schmidt and Hood 2014; Sun et al. 2014), systematics and evolution (Kenney-Hunt et al. 2014; Harris et al. 2015). However, little has been reported about the biology of the species, such as the Yucatecan deer mouse Peromyscus yucatanicus, which is semi-tree-dwelling and endemic to the Yucatan peninsula, with one record in each of Guatemala and Belize (MacSwiney et al. 2012). The same is true for the Mexican deer mouse Peromyscus mexicanus, which is also semi-tree-dwelling in habit and presents the widest geographic distribution within the genus Peromyscus, stretching from San Luis Potosí, Mexico to Panama, except for the Yucatan peninsula (Trujano-Alvarez and Álvarez-Castañeda 2010). Its abundance is highest in conserved remnants of tropical medium and high forest present in states such as Veracruz, Oaxaca and Chiapas, Mexico (Sánchez-Hernández et al. 2001; Cruz-Lara et al. 2004; Cruz-Lara et al. 2010).
Considering the ecological importance of the rodents and the paucity of biological information regarding these two species P. yucatanicus and P. mexicanus, this paper studies these two species to evaluate aspects of their population structure and dynamics, characterization and microhabitat and feeding preferences in sites of tropical medium forest in the states of Quintana Roo and Veracruz, Mexico, in order to obtain basic information regarding their biology that could contribute to the conservation of both the rodents and the ecosystems they inhabit.
Materials and Methods
Study areas. The study was conducted in two localities. The first site was located within a tropical medium subevergreen forest (21° 12´ 35” N, -87° 12´ 26” W and 21° 12´ 38” N, -87° 12´ 28” W) of El Edén Ecological Reserve (EEER), in the municipality of Lázaro Cárdenas in Quintana Roo, Mexico (Figure 1). The EEER covers an area of 3,077 ha and presents an elevation range of 5 to10 masl. The vegetation types present are tropical medium subevergreen forest, wetlands with savannah, palm stands, tintales and areas of acahuales of tropical medium forest in recovery (Allen and Rincón 2003). The tropical medium subevergreen forest has an average canopy height of 10 to 12 m, with emergent trees that reach up to 15 m in height (Schultz 2003). Its most abundant species are Metopium brownei, Manilkara zapota, Lysiloma latisiliquum, Thrinax radiata, Sabal yapa, Bursera simaruba, Brosimum alicastrum and Vitex gaumeri (Schultz 2003). The climate is warm subhumid, with a dry season in winter and spring and a rainy season from June to October. Mean annual temperature is 26 ºC and mean annual precipitation is 1,200 mm (Allen and Rincón 2003).
The second site was located in a tropical medium subevergreen forest (19° 50´ 54” N, -96° 35´ 49” W and 19° 50´ 55” N, -96° 35´ 46” W) of the Zona de Protección Forestal y Faúnica Santa Gertrudis (ZPSG), in the municipality of Vega de Alatorre in Veracruz, Mexico (Figure 1). This reserve covers an area of 925 ha and has an elevation range of 400 to 900 masl (Bojorges and López-Mata 2005). The climate is semi-warm, with a mean annual temperature of 22 ºC and mean annual precipitation of 1,845 mm (Godínez-Ibarra and López-Mata 2002), with a rainy season from June to November (García 2004). The trees of the tropical forest in this study site reach heights exceeding 20 m and the species most prominent for their structural importance are Aphananthe monoica, Brosimum alicastrum, Bursera simaruba, Dendropanax arboreus, Faramea occidentalis, Protium copal, Sapindus saponaria and Tabernaemontana alba (Godínez-Ibarra and López-Mata 2002).
Data collection. To capture P. yucatanicus in the EEER, one field visit was conducted during the rainy season (September 2014) and two in the dry season (November 2014 and February 2015). A total of 122 Sherman traps (23 x 8 x 9 cm) were set at between 2.00 and 10.00 m above ground level, in 62 trees spaced equidistantly at 10.00 m apart, forming a grid of 11 columns by six rows. To capture P. mexicanus in the ZPSG, three samplings were conducted in the dry season (March, May and June 2015) and three in the rainy season (July, August and September 2015). A total of 48 trees were selected at 9.00 m apart, forming a grid of eight columns and six rows, over which 96 traps (23 x 8 x 9 cm) were distributed at between 0 and 4.50 m in height above ground level. In addition, to record captures of P. mexicanus at greater height, a trap was set at between 6.60 and 11.20 m above ground level on each of nine trees selected from the interior of the grid.
In each site, the traps remained open for five consecutive nights per sampling, and used sunflower seeds and oats mixed with vanilla as bait. Then trees selected for the traps were characterized by presenting branches and lianas that interconnected them with other individuals. The traps were fixed to the trunks and branches using metal mesh wire and an elevator system with wooden platforms, nylon strings and carabiners (techniques modified from Vieira 1998 and Graipel 2003). The capture and recapture method (Krebs 1966) was used, and the captured individuals were marked with perforations on the ears (Sikes and The American Society of Mammalogists 2016). For each captured individual, the weight, sex, age (juvenile or adult), reproductive condition (whether the males presented scrotated testicles and the females presented an open vagina, were pregnant or in lactation) and the following morphometric measurements were recorded with a digital vernier: total length (TL), tail length (TaL), right hind-foot length (HFL) and right ear length (EL). The feces of both captured species were collected from inside the traps and stored in Eppendorf tubes at -20 ºC (Vázquez et al. 2004).
Analysis of the population information. The capture effort for each species was calculated from the number of traps located in each site (122 in the EEER and 105 in the ZPSG), multiplied by the number of nights of each sampling, expressed as night/traps. The percentage of sampling success was calculated with the total number of captures in each month divided by the number of night/traps in the month. The population structure of each species was obtained considering the sex and age data of the captured individuals. From the total number of reproductive individuals, it was possible to determine the variation of the reproductive activity in each site (Rojas-Martínez et al. 2012). Mann-Whitney U tests were used to determine whether differences existed in the heights of capture between sexes per sampling period. Population density was calculated using the method of Minimum Number Alive (MNA, Krebs 1966). Contingency tables and Pearson χ2 tests were used to determine the probable dependence of the frequency of sexes, ages and reproductive condition (with the latter used only for P. mexicanus), with respect to the months and seasons of sampling. Given that, in both species, only the proportions of ages showed dependence, post-hoc Analysis of Standardized Residuals was conducted to determine which age classes were dependent. In order to evaluate whether significant differences existed in the somatic measurements between sexes, a one-way analysis of variance (ANOVA) was used for the variables with normal distribution, while the Mann-Whitney U test was used for those without normal distribution.
Characterization and preference of microhabitat. The trees in which the rodents were captured were identified taxonomically using keys (Castillo-Campos and Medina-Abreo 2005) and the help of botanical experts. Tree diameter at breast height (DBH, in cm) was recorded and the percentage of canopy openness calculated (CO, Frazer et al. 1999). Around the base of each tree, four plots of 1.00 m2 were established, each oriented towards a cardinal point. These were used to characterize the microhabitat at ground level through the percentage of vegetal coverage, organic matter (composed of leaf litter and dead wood deposited on the soil), rocky and bare soil surfaces (Poindexter et al. 2013). Characterization was conducted in February 2015 for P. yucatanicus, and in June and September 2015 for P. mexicanus. For P. mexicanus, one-way ANOVA and Mann-Whitney U tests were used to determine whether significant differences existed in the percentages of the variables between seasons. The variables coverage of grasses and cacti were not subjected to these statistical tests, since the data were insufficient to make the comparisons. Principal components analysis (PCA) with matrices of variance-covariance and generalized linear models (GLM) were used to define which variables of the microhabitat influenced the frequency of capture of both species (Dobson and Barnett 2008). For P. mexicanus, GLM were developed between seasons and sexes.
Figure1 Geographic location of the study site (black square) in Zona de Protección Forestal y Faúnica Santa Gertrudis, Veracruz (dark gray polygon) and study site (black circle) in El Edén Ecological Reserve, Quintana Roo (light gray polygon), México.
Composition of the feces. The fecal material of each species was analyzed using preparations on slides, with a 70.00 % alcohol solution and crystalline mucilage adhesive (Stafford de México, S. A. de C. V.) for mounting (Baltazar 2014). Each preparation was placed on millimetric paper under a stereoscopic microscope (Modelo CSM2-Labomed, Labomed, Inc.) and elements of vegetal (seeds, epidermis, fruit pulp and starch, fibers, roots and bark) and animal (larvae, complete insects, arthropod appendages and chitin remains) origin identified in five randomly selected fields of area 25.00 mm2 (Montenegro-Díaz et al. 1991; Peña-Ramos et al. 2009; Lanzone et al. 2012). The percentages of appearance were estimated based on the number of fields occupied by each element (López-Cortés et al. 2007). Remains of animal origin were identified through consultation of specialized literature (Triplehorn and Johnson 2005; AntWeb 2017) and specialist taxonomists. The remains of some insects, apparently consumed by P. mexicanus and found within the traps in which the rodent was captured, were collected and identified.
Contingency tables and calculations of Pearson χ2 were used to determine whether the frequencies of the elements were dependent on the months of sampling, in the case of P. yucatanicus, and between seasons for P. mexicanus. To determine which elements were dependent, post-hoc Analysis of Standardized Residuals was performed. One-way ANOVA, Kruskal-Wallis and Mann-Whitney U tests were used to determine whether differences existed in the frequencies of the elements between sexes and ages for P. yucatanicus and between seasons, sexes and reproductive conditions for P. mexicanus. Only those elements with sufficient data to make the comparisons were subjected to these statistical tests. The seeds found were assigned to different morphotypes based on their shape, color, surface texture and width (Wells et al. 2009) for which reason their lengths and widths were measured (in mm) with the software ImageJ Version 1.48 (Ferreira and Rasband 2012). The contingency tables and calculations of Pearson χ2 were used to determine whether the frequencies of the morphotypes were dependent on the sampling periods. To determine which morphotypes were dependent, post-hoc Analysis of Standardized Residuals was performed. Statistical analysis was conducted using the software STATISTICA Version 7 (Stat Soft, Inc.) and the graphic options of the statistical package VCD (Meyer et al. 2006; Zeileis et al. 2007; Meyer et al. 2015) for the software R (R Core Team 2014; RStudio, Inc.).
Results
Peromyscus yucatanicus: Population aspects. A total of 48 individuals of P. yucatanicus were captured and marked. This group comprised 26 males (54.00 %) and 22 females (46.00 %). Of these, 85 recaptures were achieved in 1,205 night/traps, with a capture success of 11.00 %. The average population density was 26.60 ind/ha (± 23.00 SD), with the highest density found in February (50.00 ind/ha). Over the entire sampling, 48 males (56.50 %) and 37 females (43.50 %) were recaptured. The greatest number of individuals of both sexes was obtained in November (21 males and 14 females, Figure 2a). The frequency of sexes was not dependent on the sampling month (contingency table of 2 x 3, ?2 = 0.875, d. f. = 2, P = 0.646). Twenty-two juvenile (52.40 %) and 20 adult (47.60 %) males, and 17 juvenile (48.60 %) and 18 adult (51.40 %) females were recorded. More juveniles of both sexes were recorded in November (15 males and eight females), while the highest number of adults was obtained in February (13 males and 10 females, Figure 2b). Only the frequencies of juvenile and adult males depended on the month of February (contingency table of 4 x 3, ?2 = 24.39, d. f. = 6, P < 0.001) (Supplementary material 1, Figure A1). Of the total number of individuals captured, 10 presented reproductive activity (seven males and three receptive females); these were present only in February. None of the somatic measurements of adult males and females (Table 1) presented significant differences between sexes: TL (F 1, 26 = 0.12, P = 0.73), TaL (F 1, 26 = 1.43, P = 0.24), HFL (U = 75, P = 0.13), EL (F 1, 28 = 1.52, P = 0.22).
Characterization and preference of the microhabitat. The rodents were captured on 41 trees with mean DBH of 19.10 cm (± 12.53 SD, range: 3.80 to 55.40 cm) and a CO of 7.75 % (± 3.71 SD). The identified tree species were: Lysiloma latisiliquum, Brosimum alicastrum, Pouteria campechiana, Bursera simaruba, Ceiba pentandra, Chrysophyllum cainito, Guettarda combsii and one further unidentified species of the family Sapotacea. The captures occurred at between 2.00 and 5.00 m in height above ground level, with an average height of 2.58 m (± 0.82 SD). Table 2 presents the mean heights per month, sex and age. In general, the population of males presented a mean capture height of 2.45 m (± 0.74 SD, height: 2.00 to 4.50 m) and the females 2.73 m (± 0.90 SD, height: 2.00 to 5.00 m), values that differed significantly (U = 1502.5, P = 0.006).
The dominant element of the microhabitat consisted of organic matter, with a proportion of 65.71 % (± 14.62 SD), followed by vegetal cover with 26.83 % (± 14.47 SD), which comprised climbing and herbaceous plants, as well as trees (Table 3). According to the PCA, the variables coverage of trees and leaf litter (Component 1) and DBH (Component 2) presented the greatest variations. Both components explained 86.67 % of the variation (Supplementary material 2, Table B1). The GLM conducted with the variables selected from the PCA showed that the vegetal cover of the trees positively influenced the frequency of capture (D 1, 39 = 6.910, P = 0.02) (Supplementary material 1, Figure A2). In contrast, leaf litter had a negative influence on the number of rodents captured of the rodent (D 1, 39 = 9.497, P = 0.008) (Supplementary material 1, Figure A3).
Composition of the feces. A total of 80 fecal samples of P. yucatanicus were analyzed (47 males and 33 females) for a total of 800 fields of 25.00 mm2. The composition of the feces was dominated by elements of vegetal origin: seeds (85.00 %), fruit pulp and starch (34.60 %), fibers (2.50 %) and different types of epidermis (0.75 %). The components of animal origin were chitin remains (0.50 %) and different types of arthropod appendages (0.10 %), including some parts of legs of the Orders Orthoptera and Coleoptera, scales of Lepidoptera and complete specimens of ants of the family Formicidae. The material that could not be identified represented 3.10 % of the total.
In both November and February, seeds were the element that presented the greatest proportion, and remains of animal origin were present only in November (Table 4). The frequencies of the elements seeds and fruit pulp and starch were dependent on the sampling month (Supplementary material 1, Figure A4). Likewise, the element of fibers was found to be dependent on the month of February (contingency table of 6 x 2, ?2 = 108.887, d. f. = 5, P < 0.001). The frequencies of the elements seeds and fruit pulp and starch, did not present significant differences between sexes (seeds: U = 657, P = 0.24; fruit pulp and starch: U = 646.5, P = 0.20) or ages (seeds: H = 3.60, d. f. = 3, n = 80, P = 0.30; fruit pulp and starch: H = 2.27, d. f. = 3, n = 80, P = 0.51). The seeds were grouped into nine morphotypes; the morphotype V was the largest in size (Length = 0.26 mm, Width = 0.26 mm), while the morphotype VIII was the smallest (Length = 0.04 mm, Width = 0.03 mm). The morphotypes I and II were mainly present in both sampling months (Supplementary material 2, Table B2). Only the frequencies of the morphotypes III, IV, V and VIII were dependent on the month of sampling (contingency table of 9 x 2,2 = 211.010, d. f. = 8, P < 0.001) (Supplementary material 1, Figure A5).
Peromyscus mexicanus: Population aspects. A total of 54 individuals were marked (27 males and 27 females), with 196 recaptures in 2,429 night/traps. Capture success was 10.30 %. The average population density was 20.66 ind/ha (± 8.70 SD), with a mean value of 14.30 ind/ha (± 5.50 SD) in the dry season and 27.00 ind/ha (± 6.24 SD) in the rainy season. Moreover, the highest record occurred in August with 32.00 ind/ha (Figure 3). A total of 95 recaptures of males was obtained: 27 in the dry season (28.40 %) and 68 in the rainy season (71.60 %). The females presented 101 recaptures: 32 in the dry season (32.00 %) and 69 in the rainy season (68.00 %). During the dry season, the month of March presented the highest number of individuals (12 males and 16 females), while in the rainy season, the highest numbers of both sexes were obtained in July (10 and 8, respectively, Figure 4a). The frequency of sexes was not dependent on the season (contingency table of 2 x 6, ?2 = 1.0, d. f. = 1, P = 0.317).
Table 1 Mean values of somatic measurements (in mm), ± standard deviations, of adult individuals of Peromyscus yucatanicus and Peromyscus mexicanus.
| Species | Sex | TL | TaL | HFL | EL |
|---|---|---|---|---|---|
| P. yucatanicus | Males (n = 15) | 194.86 ± 8.23 | 100.80 ± 5.99 | 21.05 ± 0.82 | 16.58 ± 0.70 |
| P. yucatanicus | Females (n = 13) | 195.92 ± 7.97 | 103.69 ± 6.81 | 20.53 ± 0.87 | 16.24 ± 0.81 |
| P. mexicanus | Males (n = 17) | 215.23 ± 28.16 | 124.70 ± 21.21 | 23.64 ± 0.93 | 18.10 ± 1.07 |
| P. mexicanus | Females (n = 22) | 211.22 ± 31.33 | 126.09 ± 13.44 | 23.68 ± 0.89 | 17.60 ± 0.75 |
n (number of individuals), TL (total length), TaL (tail length), HFL (right hind-foot length) and EL (right ear length)
Eleven juvenile (33.30 %) and 17 adult (60.70 %) males were captured, while the total numbers of females were seven juveniles (23.30 %) and 23 adults (76.70 %). The highest number of juveniles was presented in the dry season (nine males and six females) and no juveniles were captured in September (Figure 4b). The frequencies of juvenile and adult males were dependent on the month of March in the dry season, while those of females of both ages were dependent on the month of July in the rainy season (contingency table of 4 x 6, ?2 = 41.32, d. f. = 15, P < 0.001) (Supplementary material 1, Figure A6).
Twenty-three reproductive individuals were obtained: 11 males (47.80 %) and 12 females (52.20 %). Pregnant females were the most abundant, with 11 records (48.00 %), and these were present in all samplings (Figure 4c). The frequencies of reproductive males and females were not dependent on the season of sampling (contingency table of 5 x 6, ?2 = 3.299, d. f. = 1, P = 0.069). The mean values of the somatic measurements are shown in Table 1. None of these measurements presented significant differences between sexes, for which reason sexual dimorphism did not exist: TL (U = 183.50, P = 0.92), TaL (U = 140.50, P = 0.18), HFL (U = 185.50, P = 0.96), EL (F 1, 37 = 2.79, P = 0.10).
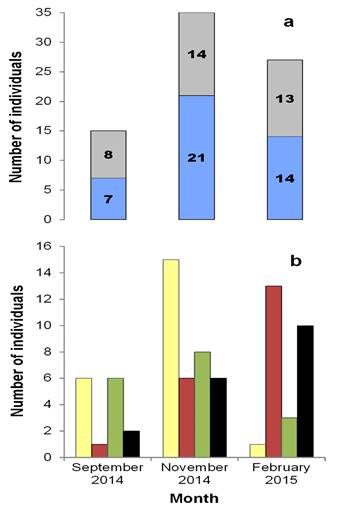
Figure 2 Structure according to: a) sex (males in blue, females in gray) and b) age (juvenile males in yellow, adult males in red, juvenile females in green, adult females in black) of the mouse Peromyscus yucatanicus in the El Edén Ecological Reserve in Quintana Roo, Mexico.
Characterization and preference of the microhabitat. In the dry season, the mice were captured in 25 trees with an average DBH of 38.62 cm (± 41.60 SD, range: 8.11 to 149.60 cm) and a CO of 4.78 % (± 2.20 SD). In the rainy season, the captures occurred in 38 trees with an average DBH of 29.98 cm (± 29.60 SD, range: 6.36 to 132.73 cm) and a CO of 4.50 % (± 2.37 SD). The tree species identified were Pimenta dioica, Psychotria sp., Pouteria sp., Spondias sp., Ficus sp., Bursera simaruba and Forchhammeria trifoliata. The captures and recaptures occurred at between 0 and 11.00 m in height above ground level, and consisted of 173 captures on the ground (14 juvenile males, 69 adult males, 15 juvenile females and 75 adult females) and 77 in the trees (3 juvenile males and 36 adult males, 3 juvenile females and 35 adult females), at an average height of 1.15 m (± 1.75 SD). In the rainy season, the juvenile males presented the greatest average capture height of 2.92 m (± 2.70 SD, Table 2), while in the dry season, the adult females presented an average capture height of 1.42 m (± 1.41 SD). In general, the population of males was captured at an average height of 1.09 m (± 1.50 SD, height: 0 to 6.70 m), while that of the females was 1.21 m (± 1.98 SD, height: 0 to 11.00 m). These values did not differ significantly (U = 5085, P = 0.97). The mean heights of capture between sexes in the dry (males = 1.18 m ± 1.55 SD, height: 0 to 4.30 m; females = 1.17 m ± 1.39 SD, height: 0 to 4.50 m) and rainy (males = 1.07 m ±1.49 SD, height: 0 to 6.70 m, females = 1.23 m ± 2.18 SD, height: 0 to 11.00 m) seasons presented no significant differences (dry: U = 360.50, P = 0.97; rainy: U = 2713, P = 0.92).
Table 2 Mean height above ground level (in meters) ± standard deviations, at which male and female individuals of different ages and of both study species were collected.
| Species | Sampling/Season | Male juveniles | Male adults | Female juveniles | Female adults |
|---|---|---|---|---|---|
| Peromyscus yucatanicus | Sep-2014 | 2.12 ± 0.21 | 2.10 | 2.44 ± 0.33 | 2.0 |
| Nov-2014 | 2.35 ± 0.12 | 2.80 ± 0.24 | 2.89 ± 0.25 | 2.95 ± 0.28 | |
| Feb-2015 | 2.20 | 2.57 ± 0.18 | 3.40 ± 0.36 | 2.48 ± 0.18 | |
| Peromyscus mexicanus | Dry | - | 1.18 ± 1.55 | 0 | 1.42 ± 1.41 |
| Rainy | 2.92 ± 2.77 | 0.97 ± 1.36 | 0.89 ± 1.35 | 1.28 ± 2.28 |
(-) no data
In both seasons, the organic matter (mainly leaf litter) was mainly present in the microhabitat, comprising 61.45 % (± 13.45 SD), followed by the vegetal cover at 29.25 % (± 13.33 SD), bare soil at 7.52 % (± 9.90 SD) and rocky surface at 1.76 % (± 3.37 SD;Table 3). The CO did not differ significantly between seasons (U = 427, P = 0.50). The percentage of climbing plants was significantly greater in the dry season (U = 212, P < 0.001), while the herbaceous plants and ferns did not differ between seasons (herbaceous plants: U = 345.50, P = 0.06; ferns: U = 471, P = 0.95). The percentage of shrubs was significantly greater in the dry season (U = 316, P = 0.02) and the coverage of trees was similar between the two seasons (U = 390.50, P = 0.23). The percentages of remains of wood (U = 460, P = 0.83) and leaf litter (F 1, 61 = 0.207, P = 0.65) did not differ significantly between seasons. The rocky surface was also similar between seasons (U = 427.50, P = 0.50) and the proportion of bare soil was greater in the rainy season (U = 198.50, P < 0.001; Table 3).
Table 3 Mean values ± standard deviations, of the variables used for characterization of the microhabitat of both study species.
| Peromyscus yucatanicus | Peromyscus mexicanus | |||
|---|---|---|---|---|
| Variable | February-2015 (n = 41) | Dry season (n = 25) | Rainy season (n = 38) | General (n = 63) |
| DBH | 19.11 ± 12.53 | 38.62 ± 41.60 | 29.98 ± 29.60 | 33.41 ± 34.80 |
| CO | 7.75 ± 3.71 | 4.78 ± 2.20 | 4.50 ± 2.37 | 4.61 ± 2.29 |
| Plant cover (%) | ||||
| Climbing plants | 3.21 ± 3.07 | 1.06 ± 0.91 | 0.30 ± 0.36 | 0.60 ± 0.73 |
| Grasses | 0 | 0.15 ± 0.48 | 0 | 0.06 ± 0.31 |
| Herbaceous plants | 0.30 ± 1.49 | 17.10 ± 13.65 | 11.48 ± 10.94 | 13.70 ± 12.30 |
| Cacti | 0 | 0.15 ± 0.48 | 0.14 ± 0.49 | 0.14 ± 0.48 |
| Ferns | 0 | 5.41 ± 7.59 | 4.16 ± 4.74 | 4.65 ± 6.01 |
| Shrubs | 0 | 2.48 ± 4.60 | 0.26 ± 0.68 | 1.14 ± 3.11 |
| Trees | 23.31 ± 13.65 | 9.40 ± 15.77 | 8.65 ± 5.69 | 8.94 ± 10.76 |
| Organic matter (%) | ||||
| Dead wood | 5.41 ± 3.42 | 4.13 ± 2.17 | 4.37 ± 2.78 | 4.27 ± 2.54 |
| Leaf litter | 60.29 ± 14.83 | 56.21 ± 13.81 | 57.81 ± 13.60 | 57.17 ± 13.59 |
| Rocky surface (%) | 3.87 ± 4.74 | 1.23 ± 2.44 | 2.11 ± 3.85 | 1.76 ± 3.37 |
| Bare soil (%) | 3.58 ± 3.55 | 2.70 ± 2.17 | 10.70 ± 11.63 | 7.52 ± 9.90 |
n (number of trees), DBH (diameter at breast height in cm), CO (canopy openness: %)
The three first components of the PCA of the rainy and dry seasons were selected. These explained 89.36 % of the accumulated variation. The variables DBH (Component 1), leaf litter (Component 2) and herbaceous plants (Component 3) presented the greatest variation (Supplementary material 2, Table B3). The GLM were conducted with the variables selected from the PCA, which showed that the interaction with the variables DBH and leaf litter had a negative effect on the frequency of capture of the rodents (D 1,58 = 5.726, P = 0.04) (Supplementary material 1, Figure A7). Moreover, the rainy season had a positive influence on the number of captures (D 1, 61 = 14.907, P = 0.001; Supplementary material 1, Figure A8). The three first components of the PCA of males and females were selected; these individually presented the variables DBH, leaf litter and herbaceous plants as those of greatest explanatory variation. The accumulated variation of the three components was 88.76% (Supplementary material 2, Table B4). The GLM produced with the variables selected from the PCA showed the interaction of DBH and leaf litter to be significant, negatively influencing the number of mice captured (D 1, 88 = 9.567, P = 0.001; Supplementary material 1, Figure A9).
Composition of the feces. A total of 132 fecal samples of the Mexican deer mouse (62 males and 70 females) were collected, of which 1320 fields of area 25.00 mm2 were analyzed. The feces contained fruit pulp and starch (66.51 %), chitin remains (51.59 %), different types of epidermis (15.00 %), seeds (4.40 %), arthropod appendages (1.70 %) and fibers (0.15 %), and 5.70 % of the material fecal could not be identified. In both seasons, the elements fruit pulp and starch as well as chitin remains were present in greater proportion. Seeds and remains of arthropod appendages were more frequent in the rainy season, while the element epidermis was more frequent in the dry season (Table 4).
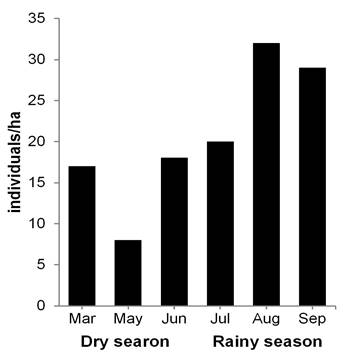
Figure 3 Population density of Peromyscus mexicanus in a tropical medium subevergreen forest of Zona de Protección Forestal y Faúnica Santa Gertrudis, in Veracruz, Mexico.
The frequencies of all of the elements of the diet were dependent on both seasons (Supplementary material 1, Figure A10), apart from fibers in both seasons and arthropod appendages in the rainy season only (contingency table of 6 x 2, ?2 = 145.049, d. f. = 5, P < 0.001). The element chitin remains presented significant differences between seasons (U = 714.5, P < 0.001) but did not differ between sexes (U = 1981.5, P = 0.39) or with the reproductive condition of the males (F 1, 54 = 0.164, P = 0.68) and females (H = 1.11, d. f. = 3, n = 25, P = 0.77). Likewise, fruit pulp and starch differed between seasons (U = 1460, P = 0.001), but not between sexes (U = 2147, P = 0.91), or male (U = 361, P = 0.91) and female (H = 1.96, d. f. = 3, n = 25, P = 0.57) reproductive conditions. The element epidermis presented similar proportions in both seasons (U = 1846.5, P = 0.16), as with between sexes (U = 2084, P = 0.69), reproductively active and inactive males (U = 337.5, P = 0.61) and among females in a reproductive state (H = 1.15, d. f. = 3, n = 25, P = 0.76).
The seeds were assigned to 24 morphotypes; morphotype number XI was that of greatest size (length = 1.31 mm, width = 0.92 mm), while number VII was the smallest (length = 0.03 mm, width = 0.04 mm; Supplementary material 2, Table B5). Remains of seeds belonging to Ficus sp. were identified in the samples from the months of May and June. The frequencies of the morphotypes I, II, III, VI and XII were the only ones that depended on the dry season (contingency table of 8 x 2, ?2 = 53.858, d. f. = 7, P < 0.001; Supplementary material 1, Figure A11).
Three seedlings of less than 2.00 mm in length were found in the feces, possibly belonging to the genus Selaginella. Some arthropod appendages corresponded to the Orders Coleoptera and Hymenoptera (some to the family Formicidae), and unidentifiable immature individuals were observed (pupae and larvae). The remains of insects collected from within the traps belonged to four individuals of the family Blattidae (Blattodea), three beetles (Coleoptera) of the family Scarabaeidae and the head of an ant (Hymenoptera) of the genus Pachycondyla.
Table 4 Proportion (%) of the elements found in the feces of Peromyscus yucatanicus during the months of sampling. The percentages of appearance were estimated based on the number of fields occupied by each element in five randomly selected fields of area 25 mm2 (López-Cortés et al. 2007).
| Month/Season | Seeds | Fruit pulp and starch | Fibers | Epidermis | Chitin remains | Arthropod appendages |
|---|---|---|---|---|---|---|
| P. yucatanicus | ||||||
| November-2014 | 78.50 | 48.50 | 3.80 | 1.10 | 0.75 | 0.20 |
| February-2015 | 98.0 | 7.40 | - | - | - | - |
| P. mexicanus | ||||||
| Dry | 1.72 | 74.0 | 0.17 | 21.20 | 30.30 | 0.51 |
| Rainy | 6.48 | 60.70 | 0.13 | 10.13 | 68.20 | 2.70 |
(-) no data
Discussion
Population structure and dynamics. The population density of a species can be affected by different factors such as climatic fluctuations, site conservation state and the availability and diversity of resources (Santos-Moreno et al. 2007; Briones-Salas and González 2016). In this study, the population density of P. yucatanicus, particularly in February (50.00 ind/ha), was similar to that observed in other localities of the Yucatan peninsula during the dry season (33 to 36 ind/ha, Cimé-Pool et al. 2007; 23 ind/ha, Hernández-Betancourt et al. 2012). The abundance of species of legumes and gramineae that produce large quantities of seeds during the dry season seems to explain the greater density of P. yucatanicus in different habitats such as agroecosystems of pasture and of the tropical forests of Yucatan (Cimé-Pool et al. 2007; Hernández-Betancourt et al. 2012). In contrast, for P. mexicanus, the estimated population density was lower (26.60 ind/ha) than that recorded in a cloud forest (34 ind/ha), but greater than that reported in an abandoned pasture (11 ind/ha) in Costa Rica (Rojas and Barboza 2007). Our results show that the rainy season presents a population increase in P. mexicanus, similar to that found in other studies (Cruz-Lara et al. 2004; Rojas and Barboza 2007; Rodríguez-Macedo et al. 2014).
Some environmental factors can influence the proportion of sexes in some species of small rodents, as is the case in Peromyscus maniculatus borealis, the proportion of which seems to be affected by precipitation causing a biased mortality among the young (Havelka and Millar 1997). For Sigmodon hirsutus, a greater mortality among juvenile females could be the cause of the existence of a greater quantity of adult males (Monge 2008). In other rodents, such as Oryzomys chapmani, it is proposed that some of their populations of tropical montane cloud forest present a greater proportion of males as a response to disturbances in their environment (Santos-Moreno et al. 2007). In this study, despite the fact that were no significant differences in the proportion of sexes of P. yucatanicus, the capture of males was slightly greater, as has been reported in other environments of tropical low forest and pasture in Yucatan (Hernández-Betancourt et al. 2012). It should be noted that the presence of fires and hurricanes is common in the state of Yucatán, for which reason future studies should evaluate the effect of these phenomena on the populations and sexual proportions of P. yucatanicus, as have been reported for other rodent species (Santos-Moreno et al. 2007). Likewise, we identified that the frequency of sexes of P. mexicanus did not depend on the season, which could indicate that neither species has suffered a selective pressure that influences the proportion of the sexes in their populations.
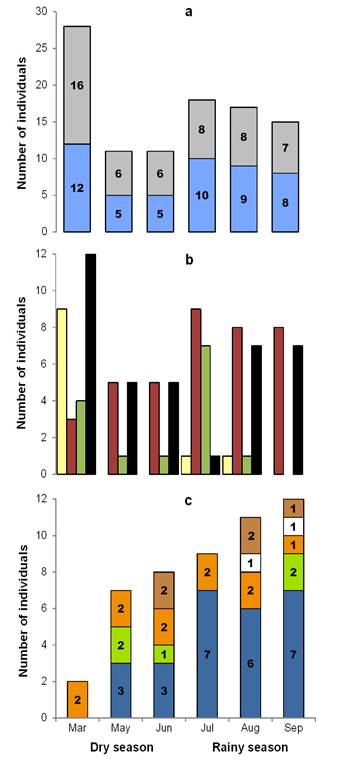
Figure 4 Structure according to: a) sex (males in blue, females in gray), b) age (juvenile males in yellow, adult males in red, juvenile females in green, adult females in black) and c) reproductive state (active males in blue, receptive females in green, pregnant females in orange, lactating female in white, postlactating females in brown) of the mouse Peromyscus mexicanus in Zona de Protección Forestal y Faúnica Santa Gertrudis, Veracruz, México.
Some species present the birth of their young in favorable periods such as the rainy season, since this is a strategy that maximizes the availability of food for the young and increases their probability of survival and maturation (Crespin and Lima 2006; Santos-Moreno et al. 2007). More juveniles of the Yucatecan deer mouse were captured in November, possibly due to the fact that most births occur at the end of the dry season and, by the rainy season, the juveniles have separated from their mothers (MacSwiney et al. 2012). We also recorded the highest capture of adults and reproductive individuals in the dry season, which coincides with another study conducted in the same area, in which receptive females were observed in the month of February (MacSwiney et al. 2012). This leads us to suggest that the reproductive activity of P. yucatanicus occurs with greater frequency at the end of the dry season and beginning of the rainy season (Hernández-Betancourt et al. 2012; MacSwiney et al. 2012), as is the case with Heteromys gaumeri in the tropical forests of Yucatan, where the number of pregnant and post-lactating females is higher during the dry season (Hernández-Betancourt et al. 2003).
In general, the number of adult P. mexicanus was usually greater than that of the juveniles. However, in March, mainly juvenile males were caught, while a greater quantity of juvenile females was captured in July. Under experimental conditions, P. mexicanus can reach adulthood 35 days after birth, regardless of sex, and reach sexual maturity at 46 to 66 days (Duquette and Millar 1995; Trujano-Alvarez and Álvarez-Castañeda 2010). This rapid maturation of the juvenile rodents could explain the difference in ages observed in our study. Moreover, the availability of vegetation with high productivity could allow the juveniles to reach adulthood in a short time (Duquette and Millar 1995; Rojas and Barboza 2007).
During the dry and rainy seasons, we observed that P. mexicanus maintained its reproductive activity, since we captured reproductive males in each month of sampling (except March), with a slightly greater abundance in the rainy season. Receptive females were also observed in both seasons and pregnant females were present in almost all samplings, with lactating and post-lactating females mainly present in the rainy season. This leads us to assume that P. mexicanus, as with other species of the same genus, is an opportunist in terms of its reproduction since in favorable years or environments, it can reproduce throughout the year (Heideman and Bronson 1993; Duquette and Millar 1995; Rojas and Barboza 2007; Ventura 2014).
Characterization and preference of microhabitat. Different species of semi-tree-dwelling rodents, such as P. leucopus, P. maniculatus and P. perfulvus, demonstrate the recurring use of the arboreal stratum in search of food, refuge in the high parts of the trees or for establishment of their nests (Graves et al. 1988; Sánchez-Hernández et al. 2009; Klein and Cameron 2012). In the case of P. yucatanicus, the present study recorded the maximum height at which it was captured at 5.00 m above ground level. It had previously been recorded at 1.65 m in a tropical medium subevergreen forest of Quintana Roo (MacSwiney et al. 2012). Our results indicate that the females can move to a greater height towards the canopy and both juveniles and adults make use of the arboreal stratum, a situation that contrasts with some records of other species, such as Ototylomys phyllotis, in which only the adults were found to use this stratum (Hernández-Betancourt et al. 2008).
With respect to P. mexicanus, one third of the total number of captures and recaptures were made on the trees, reaching a maximum capture height of 11.00 m, confirming this species as semi-tree-dwelling. The Mexican deer mouse generally establishes its burrows beneath fallen tree trunks, among the tree roots or under the ground (Trujano-Alvarez and Álvarez-Castañeda 2010), which could explain why we captured them more frequently at ground level. The average heights at which we recorded this rodent indicate that the species does not present a marked response according to sex or season in terms of climbing to the arboreal stratum. This contrast with other species of Peromyscus, such as P. perfulvus, which increase their movements on the trees during the rainy season (Domínguez-Castellanos et al. 2007).
The trees on which P. yucatanicus was captured had an average DBH of 19.11 cm, i. e., a low value if we consider that some individuals were captured in trees of up to 55.00 cm in diameter. Prominent among the tree species on which the species was captured were L. latisiliquum, B. alicastrum and B. simaruba, which are common species in the tropical medium subevergreen forest of the EEER (Schultz 2003). In contrast, Peromyscus mexicanus was captured on trees with a DBH of almost 150.00 cm; however, the average diameter of the trees on which it was captured was 33.41 cm. Bursera simaruba was a species on which P. mexicanus was captured at ground level, and one that is included among those of greatest importance for the vegetal structure of the tropical medium subevergreen forest of the ZPSG (Godínez-Ibarra and López-Mata 2002). Most of the trees on which both species were captured presented rough bark and were covered with climbing plants, which can facilitate the ascent of the rodents on the trunk (Lambert et al. 2006; Kilgore et al. 2010). Tree-dwelling and semi-tree-dwelling rodents prefer to move on arboreal substrates (trunks and branches) of small and medium diameters. This is due to morphological adaptations of the cranium and feet (in addition to the claws that help them climb vertical strata), their specialized locomotion and corporal posture, which allow them unlimited movement on narrow substrates (Graves et al. 1988; Hyams et al. 2012; Youlatos et al. 2015; Camargo et al. 2019).
The specific sites of capture for both species displayed a closed canopy since low percentages of canopy openness (7.75 % for P. yucatanicus and 4.61 % for P. mexicanus). Different authors state that tree-dwelling and semi-tree-dwelling rodents often prefer habitats with dense vegetation in both understory and canopy (Schnell et al. 2008; Pérez-Lustre and Santos-Moreno 2010; Briones-Salas et al. 2012), since these characteristics offer the advantage of avoiding detection by predators (Villanueva-Hernández et al. 2017).
Captures of both P. yucatanicus and P. mexicanus diminished in sites with a higher percentage of leaf litter. In this regard, some studies document that rodents of the same genus prefer to inhabit sites with less leaf litter and organic material, or at least to limit their movements in areas in which these are abundant (preferring to move along the length of fallen trunks and branches), with the aim of avoiding being more visually or aurally detectable by predators (Roche et al. 1999; Klein and Cameron 2012).
Composition of the feces. The dominant element in the feces of P. yucatanicus was the seeds, which comprised nine microscopic morphotypes. This indicated the likelihood that the fruits that produced these seeds are important in its diet, particularly in the middle of the dry season (February). Fruit pulp and starch was abundant at the beginning of the dry season (November), but diminished considerably by the middle of this period (February), possibly due to the lack of fruits. However, some tropical forests of the Yucatan peninsula can maintain this particular food supply throughout the year, due to succession in the production of fruits, such as those of the species Diospyros anisandra, Diospyros tetrasperma and Diospyros acapulcensis subsp. verae-crucis (Hernández-Betancourt et al. 2003; MacSwiney et al. 2012).
The elements of vegetal origin that dominated the feces of P. mexicanus were different types of epidermis, as well as fruit pulp and starch. The latter element is the most recurring during the dry season. This coincides with that reported in other studies that consider this rodent to be a consumer of a great variety of fruits, stems and leaves (Trujano-Alvarez and Álvarez-Castañeda 2010). Species of the same genus, such as P. aztecus and Peromyscus difficilis, also present a diet dominated by vegetal elements, with the consumption of fruits and stems prominent in these two species, respectively (Vázquez et al. 2004; Peralta 2015). Although seeds had a low abundance in the feces, 24 morphotypes were recorded, indicating the wide variety of resources available in the tropical forest, particularly in the dry season.
Studies of diet in small rodents rarely analyze the diversity and quantity of seeds that are ingested and defecated by these mammals (Montenegro-Díaz et al. 1991; Wells et al. 2009; Shiels and Drake 2011; Yang et al. 2018). These analyses could influence the knowledge regarding the ecological role of each rodent species as a predator and potential disperser of seeds (Yang et al. 2018), as well as providing information relating to the distribution of resources and mechanisms of coexistence among rodent species of similar sizes (Vieira et al. 2006; Wells et al. 2009). In this study, the types of microscopic seeds that pass through the digestive tract of the study species could not be identified; however, their sizes and frequencies of appearance were recorded. Of the total number of morphotypes, 70.00 % were smaller than those reported in other rodents of similar length and corporal weight to P. yucatanicus and P. mexicanus (Vieira et al. 2006).
In its feces, the Yucatecan deer mouse presented low proportions of elements of animal origin, and even then only in the month at the beginning of the dry season. The arthropods found in the feces included ants and acari of less than 1.00 mm2 in size, which could have been the result of accidental ingestion and association with the vegetal elements consumed by the rodent (López-Cortés et al. 2007; Peña-Ramos et al. 2009). In contrast, the feces of the Mexican deer mouse presented a high proportion of chitin remains, with a higher presence in the rainy season, which could be related to the fact that some arthropods are often more abundant during this season (Zavala-León et al. 2016). In this sense, it has been determined that the presence of arthropods in the diet can indicate a dietary flexibility that is advantageous in disturbed environments, or in situations where trophic resources are scarce (Vázquez et al. 2004; Lanzone et al. 2012). These arthropods also act as protein and hydric supplements that can increase the nutritional quality of the diet (Vázquez et al. 2004; Orr et al. 2015).











 nueva página del texto (beta)
nueva página del texto (beta)


