Local tales of a small, mysterious porpoise. Although the world’s smallest porpoise, the vaquita or Gulf of California harbor porpoise (Phocoena sinus), was first scientifically recognized in the 1950s, the species has likely been known to fisherfolk and Indigenous Peoples in parts of the northern Gulf of California since long before then. Interviews with local fisherfolk and long-time residents of the Gulf tell of an animal that is variously called “vaquita” (little cow), “cochito” (little pig; Brownell 1982), or even sometimes “duende” (ghost or spirit; Norris and Prescott 1961), which is likely what we know today as Phocoena sinus, although other species might also be referred to by these names. These reports, besides listing the known distribution in the northern Gulf of California, also describe the animals from much further south in the Gulf (e. g., Bahia de Los Angeles, Topolobampo; Norris and McFarland 1958; Norris and Prescott 1961), and even south to the very southern limits at the mouth of the Sea of Cortez (e. g., Islas Tres Marias, Bahia de Banderas; Norris and McFarland 1958). However, reports of vaquita further south than in the northern Gulf are likely to be incorrect (see below).
It is a sad fact that much of the history and lore of Indigenous Peoples of and near the Baja Peninsula appears to have been lost. We can imagine that the Peoples now termed Seri, Paipai, Kumeyaay, Cochimi, Cucapás, Kiliwa, and Guaycura, presently often greatly mixed with other tribes and westerners, would have wonderful recollections of a small porpoise in a vast sea (https://www.houstonculture.org/mexico/baja.html). An early unpublished attempt to investigate the vaquita and attempt to obtain a specimen, suggested that most or all of the southern reports were cases of misidentification and/or inconsistent use of the common names listed above, which are also used by some fishermen for common bottlenose (Tursiops truncatus) and common (Delphinus delphis) dolphins (Kelly 1975). The unpublished report by Kelly (1975) also suggested that two other assumptions that had been made about vaquita ecology may have been wrong: that 1) there were seasonal movements of the species south to the Midriff Islands area in summer, and 2) the species is mainly distributed in very shallow waters in and near the estuary of the Colorado River. Vaquita currently are restricted to the very upper portion of the Gulf of California, exhibit no significant migratory behavior, and occur mainly in waters about 10 to 30 m deep near submarine ridges often several kilometers from shore (Silber 1990a).
Scientific discovery by Kenneth S. Norris. Professor Kenneth S. Norris collected a porpoise skull in Spring 1950, while a graduate student at Scripps Institution of Oceanography in La Jolla, CA, studying several species of desert lizards. On that trip, he was mapping locations and habitat use of the Uma genus of lizards, that “lives on windblown sand” (see Appendix I, a draft by K. S. Norris of the discovery of vaquita in his own words). He found that first (for him) Phocoena sinus skull in the habitat of the zebra-tailed lizard (Calisaurus genus) among sand dunes occasionally swept by the amazingly forceful tides (and tidal fluxes of up to 5 m) of the upper Gulf of California, north of the little town of San Felipe. Norris (Appendix I) goes on to describe how this skull and two more collected by a colleague were used to establish them as of the Phocoena (porpoise) genus, probably most closely related to the Burmeister’s porpoise (Phocoena spinipinnis) of South America, a wonderful example of anti-tropical distribution, as heralded earlier by Hubbs (1952) and Davies (1963) for some animals and plants of the Gulf of California region. It is also possible that vaquita derived from an ancestor of the present-day Burmeister’s and spectacled (P. dioptrica) porpoises (Chehida et al. 2020; Morin et al. 2020). The Norris (Appendix I) draft is a delightful recollection of his discovery, the resulting first scientific trip in 1956 to find vaquita in nature and subsequent early studies. Details describing the vaquita as a new species are in Norris and McFarland (1958).
Curiously, in the initial draft description (Appendix I), Norris does not acknowledge the probable first scientific expedition to document and photograph vaquita in nature in 1979, organized by Norris himself (Wells et al. 1981; Figure 1, see also below), but then his recollections are in a never-before published first draft, and he likely would have elaborated as his draft developed. Since in the 1970s he advocated to us that vaquita had not been documented by scientists in nature, he may have in his draft writings accidentally confounded purported discoveries in 1956 with those that actually occurred in 1979 (Norris, Appendix I; Wells et al. 1981).
Early expeditions to find vaquita. According to Norris (Appendix I), he and colleagues sighted (probable) vaquita on their trip out of the town of San Felipe, during a short expedition in 1956. This is not represented in the published literature, but is in Appendix I. To the best of our knowledge, the first systematic survey effort dedicated to characterizing the vaquita occurred in 1979, conceived by Norris and Bernardo Villa-Ramírez, and conducted by Norris, Bernd Würsig, Randall Wells, and Benjamin López (Wells et al. 1981). Among the objectives of the survey were determining: 1) whether there was an extant population of vaquita in the Gulf, and 2) the present threats to the population. During March 3 - April 1, 1979, a survey was conducted over 1,960 km of transects through the upper Gulf, from the Rio Hardy, a distributary of the Colorado River, southward to the Midriff Islands, using a twin-engine, 7.6 m vessel. The survey recorded 206 sightings of 10 species of marine mammals, but only two were sightings that were probably vaquita (Figure 1). Both of these sightings occurred on March 10th, both involved two to three individuals, and both were in the northern portion of the upper Gulf in a region where most subsequent sightings of vaquita have occurred. The paucity of sightings was consistent with findings from subsequent surveys and indicative of the tenuous status of this species even 40 years ago. The surveys also noted extensive commercial fishing activities in the upper Gulf, and numerous carcasses of small cetaceans on the beaches near nets set perpendicular to shore.

Figure 1 This is possibly the first photo published of a vaquita in nature, on a rather placid sea, taken on 10 March 1979. Photo by R.S. Wells.
Upon reflection, while much is often made of “first discovery of”, this in general means “for science”, and almost always indicates re-discoveries of animals (or plants or other phenomena) long ago known and appreciated by local people. Vaquita were well known to the local fisherfolk of San Felipe, El Golfo de Santa Clara, and Puerto Peñasco, long before “we” western scientists came along. This kind of “science thinking” is outmoded, it seems to us, much as the “Columbus discovered the Americas” perspective seems ridiculous when one recognizes that the Americas were well-inhabited by humans long before Europeans came along.
Gillnetting for the croaker totoaba threatens the vaquita. The fate of vaquita may ultimately be linked to that of the totoaba (Totoaba macdonaldi), a large fish of the croaker family Sciaenidae and relative of the white seabass (Atractoscion nobilis). Like the vaquita, the totoaba is endemic to the Gulf of California. Spawners occur in the northern Gulf from December through May, with a peak January through March, sometimes in very large numbers (Flanagan and Hendrickson 1976).
Once exceedingly abundant in the upper Gulf of California during its winter-spring spawning, over-fishing severely depleted the totoaba population. The totoaba is presently listed as Critically Endangered on the IUCN Red List, listed on CITES Appendix 1, and is designated as Endangered under the United States Endangered Species Act. However, the IUCN classification is from 2010, so out of date and merits a new evaluation.
Records of commercial exploitation of the fish extend to at least the early 1920s (Craig 1926; Flanagan and Hendrickson 1976) or 1930s (Brownell 1982). Early totoaba fisheries were limited primarily to the export of dried swim bladders to Asian markets as an ingredient in gourmet soups and other uses (Chute 1928). Exports of the totoaba to the United States -- primarily San Francisco, Los Angeles and San Diego; also for swim bladder exportation to Asia -- were first reported as having occurred in the mid-1920s (see also Cisneros-Mata et al. 1995). Fishing villages in the upper Gulf grew rapidly in this period (Berdegue 1955); an estimated 200 fishermen, using mostly hook and line gear, participated in the fishery out of San Felipe alone in the mid-1920s (Craig 1926).
In this period, except for some local consumption of its meat, Craig (1926) reported there were “still many fish left to rot after their swim bladders have been removed, as the primary object of the fishery is still the manufacture of ‘buche’ (swim bladder)”. At that time, swim bladder material was sold in Chinese markets for U.S. $1.50-2.00 per pound. A market for its meat soon developed.
Initially the totoaba was harvested using spears from small boats and hook and line (Berdegue 1955; Flanagan and Hendrickson 1976). Gillnets were in use in the upper Gulf by the 1940s (Vidal et al. 1999), which may have accounted, at least in part, for an explosion of totoaba landing levels in the 1930s and 1940s (Arvizu and Chavez 1972; Flanagan and Hendrickson 1976). More than 1,200 metric tons (mt) were harvested in all but one year from 1935 through 1946; landings exceeded 2,000 mt annually in two of those 12 years (Rojas-Bracho et al. 2013; Figure 2). Judging by landing levels for the totoaba and in other fisheries using gillnets, bycatch of vaquita must also have been high in this period.
By the 1960s, the fishery expanded to even more efficient nylon gillnets. These nets consisted of a stretch-mesh size of 25 cm and were generally used at a height of 4.5 m and were 100-200 m long (Flanagan and Hendrickson 1976). These were mesh sizes that are fully capable of entangling dolphins, porpoises, and sea turtles. Such nets are especially dangerous for porpoises in the family Phocoenidae, which often seem to have difficulty avoiding them (Jefferson and Curry 1994). Following the introduction of gillnet fishing, totoaba landings exceeding 500 mt occurred each year from 1960 to 1970 (Flanagan and Hendrickson 1976; Figure 2 5). But by this time a depletion of totoaba numbers from over-fishing was probably well underway (Arvizu and Chavez 1972). Fishermen working in the community of El Golfo de Santa Clara alone had landings (in March, during peak spawning period) of totoaba that exceeded 50 mt each year from 1964 to 1970. Yields were over 200 mt in March 1964, 1965 and 1968 (3).
Over-fishing of the species has led to protection measures by the Mexican Government. Among these, as reported by Rojas-Bracho and Reeves (2013; Table 1), was a ban on totoaba fisheries established in 1975 (Flanagan and Hendrickson 1976), followed by another, banning totoaba gillnets in 1993. However, it is widely recognized that fishing in defiance of these restrictions often continued unabated by regulations (Rojas-Bracho and Reeves 2013; Taylor et al. 2017). One of us (GKS) observed illegal fishing activities numerous times in the mid-1990s, despite existing prohibitions, and another of us (TAJ) observed multiple cases of illegal fishing in 2006, 2008, 2010, 2013, 2015, and 2019. The IUCN Cetacean Specialist Group regularly summarizes such illegal net setting and results relative to vaquita (https://iucn-csg.org/vaquita/, accessed 13 March, 2021).
To our knowledge, first mention in the scientific literature of the taking of vaquita incidental to upper Gulf fisheries was by Norris and Prescott (1961), as well as by Norris (Appendix I). They described occasions, as reported to them by local fishermen, of vaquita being caught in the totoaba fishery, by trawlers (that were setting gillnets), and in nets set near beaches. Some of these incidents presumably occurred in the 1950s or before. Therefore, we believe substantial vaquita bycatch levels almost certainly accompanied gillnet fisheries for the totoaba and other species in this period before the species was even described scientifically.
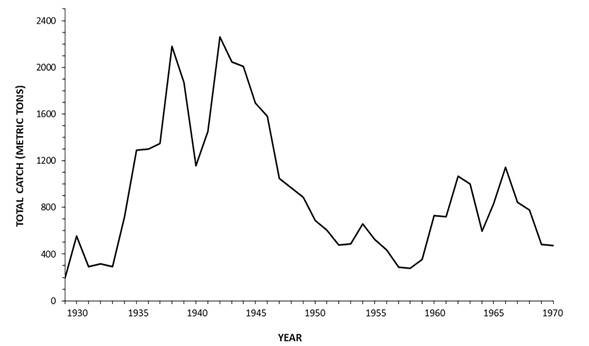
Figure 2 Yield of commercial totoaba (Totoaba macdonaldi) fishery, northern Gulf of California, 1929-1970 period. Figure modified from Arvizu and Chávez, 1972.
Dedicated studies clarify the vaquita’s endangered status. Renewed interest in the study and conservation of the vaquita occurred in the mid-1980s. Key among these were studies by Omar Vidal and Alejandro Robles, then graduate students at the Instituto Tecnológico y de Estudios Superiores de Monterrey-Guaymas. As a result, new information emerged on the totoaba fishery and apparent low vaquita abundance (e. g., Findley and Vidal 1985; Vidal 1993). These researchers helped focus attention on vaquita bycatch rates in various upper Gulf fisheries. For example, Robles et al. (1987) reported that 14 vaquita (four adults, nine calves, and one neonate) were bycaught in just three months in 1985 and 1986. Concerns were raised about the level of this bycatch and its impact on an already depleted population (Villa-Ramirez 1990, 1993).

Figure 3 The sea bass totoaba (Totoaba macdonaldi) in a fisher’s boat. The white tissue under the knife is of a recently-extracted totoaba swimbladder. Photo by G.K. Silber.
In 1986-1989, Silber (1990a, b) conducted more than 1,700 km of surveys from a small boat and more than 1,500 km of surveys from an aircraft. The surveys yielded nearly 60 vaquita sightings representing an estimated total of 110 individuals sighted (Silber 1990a, b; Silber et al. 1994). Overall, sighting rates were low (approx. two sightings per 100 km surveyed). Nearly all sightings were concentrated in a relatively small area in the northwestern Gulf (Silber 1990b). No vaquita were seen during aircraft surveys conducted along the eastern Baja California Sur peninsula south to about 29o 34’ N latitude (Silber 1990a; Silber and Norris 1991), but then aerial surveys are not optimal for sighting vaquita (Barlow et al. 1997). These findings suggested a range that might not have exceeded several thousand square kilometers.
Additional information about the species’ ecology, behavior, and affiliation with certain habitat features began to emerge from the 1980 studies. For example, ‘conventional wisdom’ at the time indicated that vaquita inhabited extremely shallow waters close to shore (see Kelly 1975). In contrast, new findings indicated that vaquita occurred primarily in water depths of 10 to 30 m, at times ten or more kilometers offshore. These were areas characterized by moderate to strong currents (although still high turbidity) driven by strong tidal surges (Silber 1990a).
Habitat partitioning among several odontocete species occurring in the upper Gulf was posited (Silber 1990a). Among other things, bottlenose dolphins and vaquita were never seen in the same vicinity. Competition between or perhaps active exclusion of vaquita by the larger-bodied bottlenose dolphins from certain very shallow habitats appears likely (see Cárdenas-Hinojosa et al. 2020). Moreover, bottlenose dolphins were found almost exclusively in (often very) shallow waters close to shore, including in marshes well into the mouth and channels of the Colorado River delta (Silber et al. 1994; Silber and Fertl 1995).
In addition, the vaquita appeared (anecdotally) to favor ‘surface slicks’ (Silber 1990a), a phenomenon resulting from mixing in the water column and water movement accompanying tidal ebb and flow and near submarine ridges. At these times, vaquita may have been feeding along underwater fronts, i. e., ‘internal waves’, resulting from moving and highly mixed water masses, again driven by substantial tidal flow. A similar use of internal waves has been reported in other cetacean species such as the harbor porpoises (Phocoena phocoena) in Monterey Bay, California (Silber and Smultea 1990) and pygmy killer whales (Feresa attenuata) in Hawai (Pryor et al. 1965).
Also occurring in the 1980s and 1990s, attempts were made to assess vaquita bycatch death rates by accompanying fisherfolks during net sets and retrievals (D’Agrosa et al. 2000), rates of recovery of net-caught vaquita (e. g., Vidal 1991), and through interviews with local fishers who were asked to recollect when vaquita were entangled (D’Agrosa et al. 2000; Turk-Boyer and Silber 1990). Estimated averages of the number of bycaught vaquita in artisanal gillnet fisheries included 15.3 individuals per year (100 between 1985 and 1990; Vidal et al. 1993), 32.3 per year (Turk-Boyer and Silber 1990), 78 per year (D’Agrosa et al. 2000), 30 to 40 per year (Vidal 1991), 58 per year (Rojas-Bracho and Taylor 1999), and tens to hundreds per year (in the 1970s; Brownell 1982, 1983). The D’Agrosa et al. (2000) estimate is based on statistical analysis, and the Rojas-Bracho and Taylor (1999) estimate is based on an experimental totoaba fishery, and these are likely the most reliable estimates in this list.
Studies of increasing sophistication ring the alarm bell. In the late 1990s and into the 2000s, research on vaquita occurrence and distribution became ever-more sophisticated, with visual sighting by 25-power “big eye” binoculars from large ships, limited hydrophone arrays for acoustic detections towed behind sail boats, and passive acoustic monitoring devices attached to the seafloor near suspected vaquita habitat (summarized in Gerrodette et al. 2011; Rojas-Bracho et al. 2019). The realization that vaquita could be identified and tracked as individuals from natural markings (Jefferson et al. 2009) provided new options for studying their biology. As a result of such photo-identification work, it was discovered that vaquita females were capable of annual calving, something which had previously been thought not possible for this species, although it has been documented for other phocoenid species (Taylor et al. 2019; see also below). This gave new hope for the species’ survival prospects.
We do not know the historical population size of the vaquita, from the period before gillnet fisheries began taking a toll on the population. The only such information comes from statistical modeling using recent estimates from vessel surveys and acoustic data, and back-calculating by incorporating information on known bycatch levels. This exercise suggests that the pre-exploitation population of the species was likely <5,000 individuals (Jaramillo-Legorreta 2008; Table 1). From analysis of the full genome, around 5,000 for around 200,000 years ago has also been estimated (Morin et al. 2020). As might be expected for a species with such a small and confined range, the vaquita has never been an abundant species. The earlier referenced extensive survey in 1979 (Wells et al. 1981), for example, yielded very low sighting rates relative to the survey area covered, evidence of a low abundance even at that time.
So, although the vaquita has always been thought to be a species with low abundance, there were no statistically defensible estimates for the species until the late 1980s. Barlow et al. (1997) presented the first estimates of abundance made from various methods, but all based on surveys that were somewhat compromised or incomplete (Table 1). The estimates also had high CVs, ranging from 39 to 143 %, and therefore had to be considered very approximate. These estimates did, however, show both that the population was small, in the hundreds (224 to 855), and that the species was declining (Table 1).
Table 1 Numerical population estimates made for the vaquita. Note that the estimates in Barlow et al. (1997) are very imprecise and potentially biased and therefore should not be taken as evidence of an increase in numbers.
| Time Period | Estimate | Conf. Int. | %CV | Reference |
|---|---|---|---|---|
| Historical | ca. 5,000 | 2,088-10,697 | nd | Jaramillo-Legorreta 2008 |
| 1986-1988 | 503 | 163-1,551 | 63 | Barlow et al. 1997 |
| 1988-1989 | 855 | 340-2,149 | 50 | Barlow et al. 1997 |
| 1991 | 572 | 73-4,512 | 143 | Barlow et al. 1997 |
| 1993 | 224 | 106-470 | 39 | Barlow et al. 1997 |
| 1997 | 567 | 177-1,073 | 51 | Jaramillo-Legorreta et al. 1999 |
| 2008 | 245 | 68-884 | 73 | Gerrodette et al. 2011 |
| 2015 | 59 | 22-145 | 50 | Taylor et al. 2017 |
| 2016 | 30 | 8-96 | nd | Thomas et al. 2017 |
| 2018 | <19 | 6-19 | nd | Jaramillo-Legorreta et al. 2019 |
In the late 1900s and early 2000s, three ship surveys were conducted, which covered the entire range of the species, and therefore provided complete estimates using consistent state-of-the-art methods. These estimates ranging from 567 in 1997 to 59 in 2015, confirmed with high confidence that the vaquita population was in the low hundreds, and was declining at a very rapid rate (see Table1 for statistical summaries).
In recent years, a moored passive acoustic monitoring (PAM) array of hydrophones has been used to determine trends in abundance, and these trends have been compared to abundance “anchor points” from the vessel surveys described above to determine abundance in intervening years. Bayesian statistical methods have been employed to increase precision, thereby reducing the uncertainty of the estimates. Using these methods, vaquita abundance was shown to have declined from an estimated 59 individuals in 2015 to <19 in 2018 (Table 1). A recent estimate suggests that there may have been fewer than 19 individuals surviving by 2018 (Jaramillo-Legorreta et al. 2019), but there is at least the possibility that this estimate was biased downwards, especially if there are individuals outside the survey study area.

Figure 4 Progression of photographs from the 1980s through the 2000s, with modern digital photography and rapid automatic focusing of images (Figure 4b-d) portraying this cryptic species as never before. The photo with Roca Consag (Figure 4d) is especially iconic for the authors, as this is near where we have seen “the most” vaquita over our lifetimes, over a deeper sea than in surrounding areas, as mentioned by K.S. Norris, Appendix I. Photos by G. K. Silber (Figure 4a) and T. A. Jefferson (Figure 4b-d).
High bycatch rates have probably persisted for decades; reported declines in the past two decades or so likely are not new phenomena. It is possible that animals outside the survey area were not counted, and that earlier estimates of abundance were simply too low. However, recent molecular studies appear to have confirmed that the vaquita has persisted at relatively low population levels for a very long time (Morin et al. 2020). One implication of this finding is that it is possible that harmful genetic alleles have to some degree been purged from the vaquita genome, thereby increasing the species’ ability to at least partially avoid “inbreeding depression” problems often associated with very small populations. This means that it may not be too late for the species to make a recovery! However, such a positive scenario is only possible with immediate cessation of gillnet fishing in waters where vaquita occur.
Some conservation attempts. It was early on realized that bycatch in fishing nets was likely the main problem for vaquita (Norris and Prescott 1961), and Rojas-Bracho and Taylor (1999) systematically eliminated worries about large-scale habitat degradation, pollution, and low genetic diversity as major causes of decline (see also Gulland et al. 2020). Although it is not our intention here to list all conservation efforts (Rojas-Bracho and Reeves 2013 and other documents provide a thorough treatment of these efforts), a major one was the establishment of a Refuge for the Protection of the Vaquita in 2005 (Gerrodette and Rojas-Bracho 2011). A large part of this eventually-unsuccessful program relied on paid support to fisherfolk and fishing communities to curtail large-mesh netting for totoaba (Rojas-Bracho and Reeves 2013). In 2008, a Species Conservation Action Plan for the Vaquita: An integrated strategy of management and sustainable use of marine and coastal resources in the upper Gulf of California (shortened to PACE-Vaquita) was promulgated by the Mexican government. Its major aims were to ban gillnet fishing in updated protected areas believed to be vaquita relative “hot-spots”, promote alternative fishing techniques, and provide compensation to fisherfolk that abided by fishing bans (Gerrodette and Rojas-Bracho 2011). Although there were some early apparent successes, this too did not halt or reverse declines of vaquita. Taylor and Rojas-Bracho (2017) provided an overview of the species’ conservation status in the IUCN Red List of Threatened Species, as also in Taylor and Rojas-Bracho 2020). In 2017, a major international ex situ conservation effort, under the direction of the Mexican government, was launched to attempt to remove vaquita from the dangers caused by continued exposure to gillnets, and maintain them under human care until such time as their natural habitat was safe for them to be returned (Rojas-Bracho et al. 2019). However, the team decided to suspend capture efforts after catching two porpoises. A juvenile was released as it appeared stressed, and an adult female died of capture myopathy (Rojas-Bracho et al. 2019).
The Present Situation. The tenacity of the species to survive should not be underestimated, and this is a very strong incentive for us to not give up hope. But, we must also remember that a beleaguered species can “blink out” very suddenly. Among cetaceans, this may have been the case for the baiji (Lipotes vexillifer), which appears to have already become extinct when the first systematic and complete survey of its entire range was conducted in 2006 (Turvey et al. 2007).
In mid-2019, the vaquita was approaching extinction, with estimates of abundance of no more than 19 individuals (Jaramillo-Legorreta et al. 2019), population decline rate at about 50%/year, and illegal gillnet fishing was still rampant in the species’ range. The outlook does not look good, but there is still some glimmer of hope, as indicated by the most recent CIRVA report (CIRVA 2019), and the recent genetic findings (Morin et al. 2020). The statement in this report, that extinction for the vaquita is only months to a few short years away (if nothing changes), may be accurate. However, the remaining vaquita appear to spend much of their time in a very small area (a Zero Tolerance Zone for illegal fishing, which, if properly protected, could form an effective refuge), and are still reproducing (a fact, born out by the sighting of multiple newborns in 2019). Some of the remaining individuals bear evidence of surviving previous entanglements, based on dorsal fin scars (Taylor et al. 2019). And finally, we have realized that vaquita can give birth annually (Taylor et al. 2019), a major discovery, since previously we assumed that all females had a minimum of a 2-year inter-calf interval (see Hohn et al. 1996).

Figure 5 Two vaquita surface near an illegal gillnet vessel retrieving its net, one of many observed during the most recent survey for the vaquita, in the fall of 2019. Photo by Diego Ruiz, Museo de la Ballena y Ciencias del Mar.
There are studies of other small and threatened odontocete cetaceans that may inform our interpretations of how best to help vaquita. One of the best studied is a little dolphin in South America. The franciscana (Pontoporia blainvillei), a species ecologically-similar to the vaquita by also occurring in medium depth waters not far from shore, is also threatened by human activities throughout its range, and continues to be taken at rates leading to unsustainable declines in abundance. Franciscanas are found in shallow coastal waters of southern Brazil, Uruguay, and Argentina, where they are exposed to artisanal fishing nets and coastal development. In the absence of empirical data, initial international management schemes suggested the existence of only four large management units across the species range (Bordino et al. 2008). In contrast, subsequent telemetry and genetic studies have determined that franciscanas instead live in small, local populations with definable home ranges, and occur in social groupings that may have much bearing on the reproductive potential for the populations (Mendez et al. 2010; Wells et al. 2013; Wells et al. in review). Concentrated removals from these small population units can have dire consequences on their continued existence. It would be good to have similar data on social groupings and behaviors of vaquita to help gauge severity of random killings due to net entanglements on their social structure and reproductive capabilities, but it is likely too late to obtain such information on the few vaquita alive at this writing.
Concluding thoughts… and a potential sign of hope. One truth apparent from the vaquita story is that species can sometimes surprise us with their ‘tenacity’ to survive even when projections based on best available data may suggest the opposite trend. Jaramillo-Legorreta et al. (2007), in their paper in Conservation Biology, justifiably attempted to raise awareness of the severity of the vaquita issue. The authors stated that vaquita were in imminent danger of extinction. They were correct, although the timeline is now extended towards the present, possibly because of elevated recruitment due to recently-recognized annual calving rates (Taylor et al. 2019).
Recent reports also highlight the urgent need for action, such as by CIRVA (2017), Taylor et al. (2017), Thomas et al. (2017), and Taylor and Rojas-Bracho (2020). Concerns expressed in those reports are not unlike those raised decades earlier by Norris and Prescott (1961), Villa-Ramirez (1976, 1993), Robles et al. (1987), Vidal (1995) and others. Importantly, the paper by Jaramillo-Legorreta et al. (2007)succeeded in catalyzing concern for the species and helping to jump-start needed initiatives, including the 2008 large vessel survey, which provided convincing evidence of the rapid rate of decline that gillnet fishing was causing. See also Jaramillo-Legorreta et al. (2019) for an update.
Vaquita are critically endangered by one threat, entanglement in shrimp and finfish gillnets; particularly, in the past 8 to 9 years with bycatch in gillnets due to capture of totoaba. But the human societal hurdles to overcome are several. To switch from gillnets to alternative ways of fishing and perhaps alternative ways of living, requires not only good governance and enforcement of existing laws, but also buy-in by stakeholders at all levels. A lucrative, well-organized criminal element that subjugates the laws with extensive illegal fishing and corruption in societal and political sectors must also be addressed (Bessesen 2018 provides a general overview). What would make the greatest difference now is cessation of all gillnet fishing throughout the vaquita range, but in a manner that does not destroy the livelihood of fisherfolks, their families, and local economies. It is our strong impression that the Mexican government has not adequately enforced existing laws and this has allowed “bad actors” to set nets illegally again and again; see also Rojas-Bracho and Reeves (2013) and Taylor et al. (2017). One important avenue besides enforcement of fishing methods, is to cut off the trade of swim bladders at all levels, at towns and cities of the Gulf of California, international borders, and the marketing venues in Asia. Although ex situ establishment of a small breeding population might have been a possibility at some point, it is likely too late for that (Taylor and Rojas-Bracho 2020), and debatable whether it would be an appropriate effort to pursue for this species, given what was learned by the attempt in 2017 (Rojas-Bracho et al. 2019). See also Brownell et al. (2019) for an up-to-date description of bycatch in gillnets relative to endangered cetaceans. The most immediate threat to the continued existence of vaquita is entanglement by gillnets. This threat must be stopped. It is not only the vaquita that are critically endangered. There are many other populations, species, and ecosystems of our oceans. We need to keep hope alive, and have well-thought-out avenues for realizing potential ways to preserve species and their ecosystems (see for example Bearzi 2020; Jefferson 2019; Notarbartolo di Sciara and Hoyt 2020; Safina 2020; Würsig 2020). Gillnets and other fishing gear are the most immediate but not the only threats.
The relentlessly advancing certainty of climate change is likely threatening vaquita (Silber et al. 2017; although we do not have direct data for this), fisherfolks’ livelihoods, and all of Earth’s ecosystems on sea and land. We, as a species, must do a much better job of recognizing and ameliorating these very real threats to our planet’s biological diversity, and ultimately to our own survival.











 nueva página del texto (beta)
nueva página del texto (beta)


