Introduction
Jaguars Panthera onca (Linnaeus, 1758) are the largest cats in the Americas. In the northern hemisphere, their main populations are distributed in the north and south of Mexico (for example in Sonora and in the Sierra Madre de Chiapas), as well as in the north of Guatemala and Belize. In South America, the most important populations are located in the Brazilian Pantanal and in the Amazon rainforest of Colombia, Ecuador, Peru, Venezuela, Bolivia, and Brazil (Isasi-Catalá 2013; Payán et al. 2013; Espinosa et al. 2016; Maffei et al. 2016; De la Torre et al. 2017, 2019). Jaguars are top predators and therefore play a fundamental role in the dynamics of the ecosystems in which they live (Seymour 1989; Currier 1983). Specifically, jaguars contribute significantly in the top-down regulation of Neotropical food chains, because they regulate the abundance of secondary and primary consumers, which, in turn, regulates the abundance of producers, modifying the complete species assemblage in the ecosystems where they occur (Estes et al. 2011).
Because the jaguar is a species of Neotropical affinity that prefers dense forest environments with high levels of humidity, its populations have decreased dramatically in some of the driest areas of its distribution (southeastern United States, eastern Brazil and the plains of Argentina; De Azevedo et al. 2016; Di Bitetti et al. 2016; Pereira-Garbero and Sappa 2016). Currently, the Selva Maya (SM), located in the north of Mesoamerica (southeast of Mexico, north of Guatemala and northwest of Belize) is one of the highest priority jaguar conservation areas since it harbors the second largest population in the continent. In Guatemala, The Maya Biosphere Reserve (MBR) is the highest priority area for conservation of jaguars. The SM which includes the MBR has been identified as a Jaguar Conservation Unit (JCU), which means is one of the most important areas for jaguar conservation and that includes strict protection zones (Zeller 2007; García-Anleu et al. 2016; De la Torre et al. 2016, 2017; Jędrzejewski et al. 2018).
In the MBR, the aguadas are water ponds supplied with rainwater which are formed by isolated depressions on the land in which the soil is clayey and compact (Reyes 2009; Reyna-Hurtado et al. 2010; García et al. 2018). The aguadas provide a valuable resource for a variety of wildlife species, particularly during the dry season (Reyna-Hurtado et al. 2010; García et al. 2018). For predators such as jaguars, the aguadas represent a determining factor for home-range delineation, because in addition to providing drinking water, they favors the seasonal aggregation of prey that also visit these bodies of water (Muckenhirm and Eisenberg 1973; Núñez et al. 2002; Simá et al. 2008).
Home-ranges of female and male jaguars are different (Sunquist and Sunquist 2002). Females generally occupy home-ranges which include a sufficient number of prey for themselves and their cubs, while males occupy larger home-ranges, maximizing access to females and maintaining sufficient prey for individual survival (Schaller and Crawshaw 1980). As a consequence, the home-range of a single male jaguar is occupied by the home-ranges of several females (Astate et al. 2008; Morato et al. 2016; McBride and Thompson 2018).
The availability of surface water and the abundance and availability of prey are factors that largely determine the patterns of habitat use by jaguars (Sunquist 1981; Mizutani and Jewell 1998; Scognamillo et al. 2002; Figel et al. 2019; Rabelo et al. 2019). In most of the MBR and the SM, the availability of surface water during the dry season is mostly restricted to the aguadas. In this study, we described the patterns of visitation to aguadas by jaguars in the Dos Lagunas Protected Biotopo (DLPB), a core zone of the MBR, through visitation rates (VR) and activity patterns (AP) during the dry season.
Material and Methods
Study site. The DLPB, located in the extreme north of Guatemala, has an extension of 307 km2. It borders the Mirador-Río Azul National Park in the east and west, the Calakmul Biosphere Reserve in Mexico to the north, and the Multiple-use Zone of the MBR to the south. It is one of the protected areas with least human impact in Guatemala and is considered part of the “Heart of the Selva Maya” (CONAP 2015; Veras 2009). The DLPB is characterized, like the rest of the MBR and the SM, by karstic and permeable soils which result in an underground drainage. The lower areas of the forest have the highest percentage of clayey soil and less permeability, favoring the formation of water ponds that store rainwater such as the aguadas (Araujo 2014; Veras 2009; Reyna-Hurtado et al. 2010; García et al. 2018).
Aguadas are usually found in low densities; they are scattered in the landscape and can vary in size, ranging from 236 m2 to several hectares (González 2015; Reyna-Hurtado et al. 2010). Since the aguadas are formed by the accumulation of rainwater, their dynamics is strongly associated with the intensity and duration of the dry and rainy season of each year. Some aguadas can remain filled through the year, and some tend to dry out completely during the dry season (González 2015). Due to the low availability of surface water in the region, the aguadas are one of the main sources of water for wildlife during dry periods, especially where large bodies of water are non-existent and humidity is low (Simá et al. 2008; Reyna-Hurtado et al. 2010; García et al. 2018).
Camera-trapping. Photographs obtained from the San Carlos University of Guatemala (Usac), Centro de Estudios Conservacionistas (Cecon) and the Central American Tapir Monitoring Program of the Defensores de la Naturaleza Foundation (FDN) were used to register jaguar activity in the aguadas of the DLPB. We monitored seven aguadas by setting up Bushnell® Trophycam camera-traps during the 2014 and 2015 dry seasons, programed to take three photographs per capture event and a 15 seconds interval between capture events; and setting up Bushnell® Trophycam Agressor No Glow camera-traps during dry season 2016 and 2017, programmed to take one photograph per capture event with a one second interval between capture events. The number of aguadas sampled and camera-traps used varied according to the year of sampling: 2 aguadas and 6 cameras in 2014; 3 aguadas and 9 cameras in 2015; six aguadas and 17 cameras in 2016; and, six aguadas and 10 cameras in 2017. Total sampling effort was 4,587 trap-days (Tables 1 and 2; Figure 1).
Table 1 Characteristics of aguadas from Dos Lagunas Protected Biotopo, Maya Biosphere Reserve, Guatemala.
| Aguada | Seasonality | Forest type | Distance to nearest aguada (km) |
|---|---|---|---|
| A1 | Seasonal | Tall | 4.9 (A6) |
| A2 | Seasonal | Tall | 1.7 (A7) |
| A3 | Permanent | Tall | 3.2 (A4) |
| A4 | Permanent | Tall | 3.2 (A3) |
| A5 | Permanent | Short | 5.3 (A1) |
| A6 | Permanent | Short | 4.9 (A1) |
| A7 | Seasonal | Short | 1.7 (A2) |

Figure 1 Location of aguadas of the Dos Lagunas Protected Biotopo, Maya Biosphere Reserve, Guatemala.
We used Camera Base version 1.7 software (Tobler 2015) to process photographs and organize them in a database with the following information: site, date, time, camera-trap station (aguada), sex and age of individuals. We identified individuals by comparing rosette patterns of the coat (Karanth and Nichols 1998). Each capture event was considered independent of another capture event if 1 hour elapsed between capture events of the same individual in the same aguada.
Visitation rates (VR). To account for differences in sampling effort, we determined visitation rates (VR) of jaguars (for females, for males, and both) using the following equation: VR = (N/SE) x 1,000 trap-days [N = number of independent captures, and SE = sampling effort measured as the number of days multiplied by the number of camera-traps in each aguada]. We calculated VR for each aguada and for all aguadas combined.
Activity patterns (AP). The activity pattern of each jaguar was classified as diurnal, nocturnal or crepuscular activity. The initial time of each visit event was used to determine these visiting patterns. Capture events recorded from one hour after dawn (sunrise) to one hour before dusk (sunset) were considered as diurnal; events recorded between one hour after dusk to one hour before dawn were considered as nocturnal; and, events recorded between one hour before dawn and one hour after dawn, and between one hour before dusk and one hour after dusk were classified as crepuscular (Monroy-Vilchis et al. 2011; Jiménez et al. 2010). The time of sunrise and sunset for each day was determined using the solar calculator from the Global Monitoring Division of the Eastern System Research Laboratory of the National Oceanic and Atmospheric Administration of the U. S. Department of Commerce (NOAA 2014).
We used the methodology of Jiménez et al. (2010) to determine the AP of jaguars in aguadas: “diurnal” when < 10 % of events were recorded at night; nocturnal when > 90 % of the visit events were recorded at night; “mostly diurnal” when night time events ranged between 10 to 30 %; “mostly nocturnal” when 70 to 90 % of the events were recorded at night; “crepuscular” when 50 % or more of the events were during twilight periods; and “catameral” when events occurred sporadically during the day and night.
Activity and overlap models. We tested for significant differences (P < 0.05) in AP between sexes fitting kernel density models to estimate and compare female and male jaguar activity distributions using a Wald test (tested on chi square distribution with one degree of freedom), and quantified and compared levels of activity for both sexes through bootstrapping (10,000 replicates) employing the package ‘Activity’ (Rowcliffe 2019) in software R (R Core Team 2020, R version 3.6.3). We estimated the coefficient of overlap (D = level of overlap) for female and male jaguar activity, and we fitted kernel density models to compare AP between sexes by using the capture events in each aguada employing the package ‘Overlap’ (Meredith and Ridout 2020) in software R (R Core Team 2020, R version 3.6.3). We estimated the confidence intervals (CI) between lower and upper 95 % limits in estimates of female and male activity overlap, calculated as percentile intervals from 10,000 bootstrap samples (Ridout and Linkie 2009). We plotted models not extended (extend = NULL, Meredith and Ridout 2020).
Table 2 Sampling effort in aguadas. *1 Camera deactivated from March 15 to May 31 2015; *2 Camera deactivated from May 7 to May 31 2015.
| Year | Aguada | Cameras | First | Last | Days | No Cameras | Effort | Total |
|---|---|---|---|---|---|---|---|---|
| 2014 | A3 | All | 10/04/2014 | 20/06/2014 | 71 | 3 | 213 | 420 |
| A6 | All | 11/04/2014 | 19/06/2014 | 69 | 3 | 207 | ||
| 2015 | A3 | All | 26/04/2015 | 06/07/2015 | 71 | 3 | 213 | 567 |
| A6 | All | 25/04/2015 | 01/07/2015 | 67 | 3 | 201 | ||
| A2 | Camera 1 | 27/04/2015 | 01/07/2015 | 65 | 1 | 65 | ||
| Camera 2*1 | 27/04/2015 | 01/07/2015 | 48 | 1 | 48 | |||
| Camera 3*2 | 27/04/2015 | 01/07/2015 | 40 | 1 | 40 | |||
| 2016 | A3 | All | 5/04/2016 | 30/08/2016 | 147 | 4 | 588 | 2,319 |
| A6 | Cameras 1-3 | 06/04/2016 | 27/08/2016 | 143 | 3 | 429 | ||
| Camera 4 | 12/05/2016 | 10/08/2016 | 90 | 1 | 90 | |||
| A5 | Cameras 1-2 | 06/04/2016 | 23/08/2016 | 139 | 2 | 278 | ||
| Camera 3 | 11/05/2016 | 23/08/2016 | 104 | 1 | 104 | |||
| A4 | All | 5/04/2016 | 29/08/2016 | 146 | 3 | 438 | ||
| A1 | Camera 1 | 11/05/2016 | 23/08/2016 | 104 | 1 | 104 | ||
| A7 | Cameras 1-2 | 12/05/2016 | 27/08/2016 | 107 | 2 | 214 | ||
| Camera 3 | 12/05/2016 | 25/07/2016 | 74 | 1 | 74 | |||
| 2017 | A3 | Camera 1 | 30/03/2017 | 19/06/2017 | 81 | 1 | 81 | 1,281 |
| Camera 2 | 30/03/2017 | 10/08/2017 | 133 | 1 | 133 | |||
| Camera 3 | 30/03/2017 | 12/08/2017 | 135 | 1 | 135 | |||
| A6 | All | 29/03/2017 | 10/08/2017 | 134 | 3 | 402 | ||
| A5 | Camera 1 | 29/03/2017 | 10/08/2017 | 134 | 1 | 134 | ||
| A4 | Camera 1 | 30/03/2017 | 5/08/2017 | 128 | 1 | 128 | ||
| A1 | Camera 1 | 29/03/2017 | 10/08/2017 | 134 | 1 | 134 | ||
| A7 | Camera 1 | 29/03/2017 | 10/08/2017 | 134 | 1 | 134 | ||
| Total | 4,587 |
Results
We obtained 409 photographs of jaguars corresponding to 60 independent visit events in the seven sampled aguadas (A1 to A7). Aguadas A3, A4 and A6, were the most frequented by jaguars (Table 3). We identified 14 individuals (eight males, five females and one of unknown sex) in 54 events (90 %) across five aguadas. At two aguadas we recorded six events (10 %) of individuals who could not be identified or sexed (Table 4).
From the 54 visit events with identified individuals, 17 (31.5 %) belonged to a single female; the rest of individuals were observed on one (2 individuals), two (3 individuals), three (4 individuals) or four (4 individuals) visit events (Table 4). The aguadas with the highest number of visit events with identified individuals were A6 with 16 (19.6 %), A3 and A4 with 13 each (48.1 %), A2 with six (11.1 %) and A7 with four visit events (7.4 %; Table 4). The aguadas with the highest number of visit events in a single year of study (of the 60 total events) were A4 (2016) and A6 (2017), with 12 and 9 events respectively, which represent 35 % of visit events from all the study (Table 3). The aguadas visited by the highest number of individuals were A6 (3 females, 4 males, 1 of unknown sex), A4 (5 females, 2 males, 1 of unknown sex), and A3 (3 females, 3 males, 2 of unknown sex), registering 8 individuals each.
Table 3 Visitation rates (VR) of jaguars by aguada, year and sex. F: Females, M: Males.
| Aguada | 2014 (F , M) | 2015 (F , M) | 2016 (F , M) | 2017 (F , M) | All years (F , M) |
|---|---|---|---|---|---|
| A1 | 9.6 (0, 0) | 0 (0, 0) | 4.2 (0, 0) | ||
| A2 | 39.2 (0, 39.2) | 39.2 (0, 39.2) | |||
| A3 | 9.4 (4.7, 4.7) | 18.8 (0, 18.8) | 6.8 (5.1, 0) | 17.2 (14.3, 0) | 11.7 (6.6, 3.7) |
| A4 | 27.4 (20.5, 6.8) | 23.4 (15.6, 0) | 26.5 (19.4, 5.3) | ||
| A5 | 0 (0, 0) | 7.5 (0, 0) | 1.9 (0, 0) | ||
| A6 | 19.3 (9.7, 4.8) | 5.0 (5.0, 0) | 3.9 (1.9, 1.9) | 22.4 (5.0, 17.4) | 12.0 (4.5, 6.8) |
| A7 | 10.4 (0, 6.9) | 14.9 (14.9, 0) | 11.8 (4.7, 4.7) | ||
| All aguadas | 14.3 (7.1, 4.8) | 19.4 (1.8, 17.6) | 9.5 (5.6, 2.6) | 16.4 (8.6, 19.5) | 13.1 (6.1, 5.5) |
Visitation rates (VR). We estimated a VR of 13.1 records for 1,000 trap-days for jaguars in all aguadas and all years (Table 4). However, VR varied among individual aguadas (VR = 1.9 to 39.2 records for 1,000 trap-days) and years (VR = 9.5 to 19.4 records for 1,000 trap-days), both for all jaguars combined and separately for females and males. We did not record visit events in aguadas A1 in 2017 and A5 in 2016 (VR = 0 records for 1,000 trap-days), while in aguadas A2 in 2015 and A4 in 2016 we determined the highest values, VR = 39.2 and VR = 27.4 records for 1,000 trap-days, respectively. Females showed higher VR in aguadas than males (VR = 6.1 and 5.5 records for 1,000 trap-days, respectively), with variation according to the years and the aguadas sampled (Table 3).
Activity patterns (AP). Jaguars presented a bimodal activity pattern, showing two peaks that occurred at mid-morning, and before dusk. Across all years, jaguars showed more frequency of visits (independent events) from 18:00 to 18:59 h. However, we recorded the highest density of events between 04:00 to 11:59 h (before dawn to before noon), which represented the highest peak of activity of jaguars in aguadas (Figure 2a).

Figure 2 a: Relative frequency of records and daily activity patterns of jaguars in aguadas (n = 60), black line: bimodal distribution curve. b: Frequency of records and daily activity pattern of female and male jaguars in aguadas (n = 53). Relative frequency and bimodal distribution curve (a) were calculated and plotted using ‘geom_histogram(aes(y = ..density..))’ function and ‘geom_density ()’ function of package ‘Ggplot2’ in software R.
Table 4 Jaguar individuals visit events in aguadas of the Dos Lagunas Protected Biotopo, Maya Biosphere Reserve, Guatemala. F: Female; M: Male; U: Unknown sex.
| Year/Aguada | F1 | F2 | F3 | F4 | F5 | U6 | M7 | M8 | M9 | M10 | M11 | M12 | M13 | M14 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | A3 | 1 | 1 | |||||||||||||
| A6 | 2 | 1 | 1 | |||||||||||||
| 3 | 1 | 1 | 1 | 6 | ||||||||||||
| 2015 | A2 | 3 | 2 | 1 | ||||||||||||
| A3 | 2 | 2 | ||||||||||||||
| A6 | 1 | |||||||||||||||
| 1 | 2 | 3 | 2 | 3 | 11 | |||||||||||
| 2016 | A3 | 2 | 1 | |||||||||||||
| A4 | 4 | 2 | 2 | 1 | 2 | 1 | ||||||||||
| A6 | 1 | 1 | ||||||||||||||
| A7 | 1 | 1 | ||||||||||||||
| 6 | 2 | 3 | 1 | 1 | 3 | 2 | 1 | 19 | ||||||||
| 2017 | A3 | 4 | 1 | |||||||||||||
| A4 | 1 | 1 | ||||||||||||||
| A6 | 2 | 3 | 4 | |||||||||||||
| A7 | 2 | |||||||||||||||
| 8 | 1 | 1 | 1 | 3 | 4 | 18 | ||||||||||
| Total | 17 | 3 | 4 | 2 | 2 | 1 | 3 | 4 | 5 | 3 | 2 | 1 | 3 | 4 | 54 | |
| Year/Aguada | F1 | F2 | F3 | F4 | F5 | U6 | M7 | M8 | M9 | M10 | M11 | M12 | M13 | M14 | Total | |
| 2014 | A3 | 1 | 1 | |||||||||||||
| A6 | 2 | 1 | 1 | |||||||||||||
| 3 | 1 | 1 | 1 | 6 | ||||||||||||
| 2015 | A2 | 3 | 2 | 1 | ||||||||||||
| A3 | 2 | 2 | ||||||||||||||
| A6 | 1 | |||||||||||||||
| 1 | 2 | 3 | 2 | 3 | 11 | |||||||||||
| 2016 | A3 | 2 | 1 | |||||||||||||
| A4 | 4 | 2 | 2 | 1 | 2 | 1 | ||||||||||
| A6 | 1 | 1 | ||||||||||||||
| A7 | 1 | 1 | ||||||||||||||
| 6 | 2 | 3 | 1 | 1 | 3 | 2 | 1 | 19 | ||||||||
| 2017 | A3 | 4 | 1 | |||||||||||||
| A4 | 1 | 1 | ||||||||||||||
| A6 | 2 | 3 | 4 | |||||||||||||
| A7 | 2 | |||||||||||||||
| 8 | 1 | 1 | 1 | 3 | 4 | 18 | ||||||||||
| Total | 17 | 3 | 4 | 2 | 2 | 1 | 3 | 4 | 5 | 3 | 2 | 1 | 3 | 4 | 54 |
From the 60 total visit events, 30 (50 %) corresponded to diurnal activity (19 for females, 8 for males and 3 of unknown sex and identity), 10 (16.7 %) to nocturnal activity (2 for a female, 7 for males and 1 of unknown sex and identity) and 20 (33.3 %) to crepuscular activity (7 for females, 10 for males and 3 of unknown sex and identity). Females showed greater diurnal activity (19 events) compared to nocturnal (2 events) and crepuscular activity (7 events); while males showed 8, 7 and 10 events for diurnal, nocturnal and crepuscular activity events, respectively (Figure 2b). Females showed a unimodal distribution, visiting aguadas during the day (after dawn and before dusk), while males showed a trimodal distribution, with their maximum peak at mid-morning, and two smaller peaks after dusk, and at mid-afternoon (Figure 3a). During night (after dusk and before dawn), females showed a bimodal distribution with similar peaks after 20:00 h and before midnight, while nocturnal activity of males was increasing until reaching their peak before dawn twilight (Figure 3b). In the case of twilight periods (crepuscular activity), females and males showed different and similar activity through dawn and dusk, respectively. During dawn twilight, females showed a bimodal distribution in a very short period of time (two events at 05:37 and 05:39 h, respectively), while males increased their activity throughout dawn twilight (Figure 3c). During dusk twilight, females showed a trimodal distribution, with their maximum peak at 18:00 to 18:10 h, while males showed a bimodal distribution with their maximum peak at 18:30 to 18:45 h (Figure 3d). Only 16 % of all visit events were recorded at night hours, while 50 % were recorded at daytime, classifying jaguar activity as diurnal in aguadas.
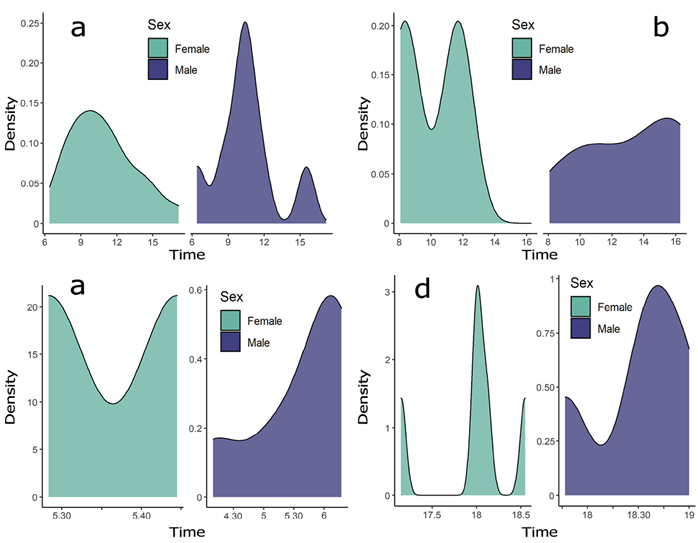
Figure 3 Activity patterns of female and male jaguars in aguadas. a: Diurnal activity. b: Nocturnal activity. c: Crepuscular activity during dawn. d: Crepuscular activity during dusk. Curves represent activity distribution density of jaguars at each data set of visit events ordered by sex. Curves were plotted using density function ‘geom_density()’ of package ‘Ggplot2’ in software R.
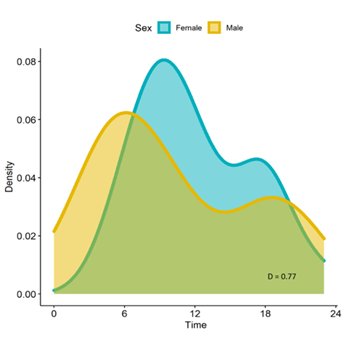
Activity and overlap models. Despite the observed differences in peaks, overall female and male jaguars did not show significant differences in their activity (P > 0.05; standard error = 0.13; Wald statistics estimate = 0.019). The estimates for observed coefficient of overlap (D = 0.65) and the mean coefficient of overlap between female and male jaguar activity (D = 0.77, 95 % confidence interval: 0.70 to 0.84), showed no significant difference between sexes in their activity (P > 0.05, Figure 4).
Discussion
The observed patterns suggest that female and male home-ranges overlap in the aguadas, with variation in the number of individuals and visit events among sites and years. For females, the recurrent use of aguadas in different years suggests a certain degree of site fidelity as reported by Cavalcanti and Gese (2009) in Brazil, where home-ranges were maintained up to 90 % for the second season, but changed the areas used more intensively. The five female individuals identified in this study were recorded in three of the seven aguadas sampled, exhibiting a high degree of home-range overlap, similar to Cavalcanti and Gese (2009) where 25 to 47 % home-ranges of female jaguars overlapped during the dry season.
For males, individuals M7, M8 and M9, were recorded in different aguadas in each of two consecutive years, which seems to suggest less fidelity to home-ranges than females, as found by Cavalcanti and Gese (2009). Our study also documented records of multiple males visiting the same aguada during the same year, which may suggest home-range overlap between males, a phenomenon that has been reported in other studies (De Azevedo and Murray 2007; Cavalcanti and Gese 2009; Harmsen et al. 2009).
Aguadas may represent regular sites of home-range overlap for jaguars considering the important resource they provide during the dry season, when they may become the only source of water used to cool off and to hunt down potential prey. In addition, Figel et al. (2019) have demonstrated that wetlands are keystone habitats for jaguars that increase their occupancy and detection probabilities. In 2016, the driest year in this study, aguada A4 was visited by six individuals (four females and two males), which may be related with the fact that A4 was the aguada that best maintained water levels across 4 study aguadas during that sampling season in the DLPB (García et al. 2018). Jaguar distribution is strongly influenced by availability of key resources (Sandell 1989), so the spatial distribution of aguadas may be an important landscape feature that influences the movements and interactions of individuals. Home-range overlap between conspecifics of the same sex has been reported to occur mainly outside core, high-quality areas, especially between females (De Azevedo and Murray 2007; Cavalcanti and Gese 2009); and the critical importance of aguadas in the JCU of the SM may rely in that these isolated water ponds are often the only surface water source available in this landscape, resulting that several individuals that use larger areas necessarily include these aguadas in their home-range.
The number of jaguars identified in our study (14 individuals) is higher than previous studies within the MBR that used camera-traps installed on trails and roads (range 7 to 10 individuals), which may indicate the ecological importance of aguadas to jaguars and which has not been studied until now (Moreira et al. 2008; Moreira et al. 2009a; Moreira et al. 2009b; García-Anleu et al. 2015a; García-Anleu et al. 2015b).
Visitation rates (VR). According to Salom-Pérez et al. (2007), Conde et al. (2010) and Sollmann et al. (2011), female jaguars occupy smaller home-ranges, move less, and show different habitat use than males. Female jaguars do not move frequently on roads, trails, and open areas, and in previous studies, they have had lower camera-trap capture rates than males when cameras are installed near these spatial features (Salom-Pérez et al. 2007; Harmsen et al. 2010a). In contrast to this, our results showed that females in aguadas have higher capture rates compared to males, despite being represented by a smaller number of individuals (55.5 % of the males recorded). This suggests the use of aguadas is more frequent by females compared to males.
Srbek-Araujo (2018) suggests that the difference in capture rates between females and males reported in literature (higher for males and smaller for females) is probably due to the fact that females tend to avoid encounters with camera-traps deliberately. This behavior was not observed in this study. In fact, the individual with the highest number of detections was a female (F1) recorded in four different aguadas across three of the four years of study (Table 4). This indicates that instead of avoiding camera-traps specifically, female jaguars in other studies may be avoiding the habitat features where camera-traps are usually installed.
Activity patterns (AP). Jaguars exhibited primarily diurnal activity at aguadas; only 16 % of records occurred at night (Jiménez et al. 2010). This data coincides with a report by González (2015) for jaguars and pumas Puma concolor (Linnaeus, 1771) in aguadas of the DLPB during the dry season 2013. According to Fedriana et al. (1999), Gliwicz and Dabrowski (2008), and Harmsen et al. (2010b), activity patterns of predators such as jaguars are associated with times of the day when their prey species are most vulnerable to predation. González (2015) reported that many potential prey species for jaguars that visit the aguadas of the DLPB are diurnal, including the great curassow Crax rubra Linnaeus, 1758, the crested guan Penelope purpurascens Wagler, 1830, the white-lipped peccary Tayassu pecari (Link, 1795), the collared peccary Pecari tajacu (Linnaeus, 1758), and the white-tailed deer Odocoileus virginianus (Zimmermann, 1780).
Although we did not find significant differences between female and male activity (Rowcliffe 2019), and that the level of overlap in activity revealed no significant differences (Meredith and Ridout 2020), sexes do differ in their peak hours of activity. Males visit the aguadas over a longer range of hours, as they have three peaks in activity. Activity of females in aguadas is concentrated primarily from mid-morning to mid-afternoon. Males showed the most activity just before and after dawn, while females showed the most activity just before and after noon; males had another peak of activity after dusk and a small peak around midday (Figure 3).
In a recent study, Sánchez-Pinzón et al. (2020) found that jaguar visitation to aguadas is significantly associated with the activity on these water ponds of the white-lipped peccary, one of its main prey (Aranda 1994; Estrada 2008). In this suggestion, sex of jaguars was not considered (Sánchez-Pinzón et al. 2020); so if female and male jaguar visitation patterns are also different as we suggest based on their different peak hours of activity in aguadas, preferred prey species may differ between males and females, as peak hours of activity of predators are strongly associated with peak hours of activity of their main prey (Fedriana et al. 1999; Gliwicz and Dabrowski 2008; Harmsen et al. 2010b).
Body mass in jaguars largely impacts their hunting decisions (Sunquist and Sunquist 2002), and it has been demonstrated that body mass among jaguar populations reflect the body mass of preferred prey species (Hoogesteijn and Mondolfi 1996). For Central American jaguars, which exhibit mean body mass differences between sexes [41.1 and 56.1 kg for females and males, respectively] (Hoogesteijn and Mondolfi 1996), differences in peak hours of activity among female and male jaguars from the same population may suggest that both sexes are preying on different prey, at different times. However, further investigation is required to identify sex-specific preferences of prey species within jaguar populations.
Sandoval-Lemus (2020) characterized the AP of Baird’s tapir Tapirella bairdii (Gill, 1865) in aguadas of the DLPB, and found that tapirs increase their activity when temperature is high and tend to reduce it when humidity rises. He also found that tapirs tend to visit the aguadas more often during the dry season compared to the rainy season, and that use of the aguadas is negatively correlated with precipitation patterns, meaning that the number of days between visits tend to increase as rain increases. Sandoval-Lemus (2020) also found that tapirs are mainly nocturnal in aguadas (70 %), and its peaks of activity are during night at 21:00 and 02:00 h. We only have sparse detections of female jaguars around these times and no detections of male jaguars but until before dawn (Figure 3b). Given this, it is possible that tapirs may be avoiding jaguars at aguadas of the DLPB, and additional research on the interaction of these two largest terrestrial mammals of the Neotropics, based on sex-specific and climatic variables at aguadas could yield interesting results (Sandoval-Lemus 2020).
This study suggests that aguadas are a limiting feature for jaguar home-ranges, and could elucidate unknown information about the habitat use of females. Despite the fact that this study did not contemplate a specific experimental design, given that its objectives were clearly descriptive, the information gathered and described reveals the frequency with which jaguars visit the bodies of water in a landscape where surface water is seasonally available, that the dynamics of use by females and males is also different, and the fact that many jaguars visit specific aguadas across multiple years. Additional research on this topic will provide invaluable information for efforts focused on the conservation and management of jaguar populations in the MBR and the SM.
Our data cannot be used for population estimates due to the design bias, as the aguadas are specific elements in the landscape that were not sampled randomly. Nonetheless, given that aguadas are a resource visited frequently by jaguars, the monitoring of aguadas and other bodies of water should be an important part of any population monitoring study and will prove useful to related research, such as jaguar collaring projects. For aguadas specific research, we recommend larger scale studies to extend this research to be able to compare visitation rates and activity patterns of jaguars in both the dry and rainy seasons, and in different landscapes features as roads and trails. Our results provide a baseline for such studies and provide data on capture rates and activity patterns that can be compared to more typical jaguar studies that tend to be done on trails and roads. In this sense, this study contributes to our overall understanding of jaguar behavior in different habitat types, information that can be useful for conservations purposes.











 nova página do texto(beta)
nova página do texto(beta)