Introduction
It is estimated that approximately 25 % of the world’s mammal species and 44 out of 77 large herbivores are threatened with extinction, mainly because of land use change and overexploitation (Baillie et al. 2010; IUCN 2013). Despite great efforts being made on their conservation, the extinction threat prevails as anthropogenic pressures (e. g., destruction of suitable habitat) increase. Efforts such as the creation of protected areas, enhanced connectivity, reduction of human footprints and reintroduction of species are a few of the mitigation initiatives being implemented to curve the demise of endangered mammals (Margules and Sarkar 2007; Bodin and Norberg 2007; Kadoya 2009).
Drought, an extreme natural event, can have devastating impacts on animal populations and it is a potential factor in species extinction (Duncan et al. 2012). The Intergovernmental Panel on Climate Change (IPCC) reported a likely increase in droughts over the 21st century in various regions of the world, including southern Europe and the Mediterranean, central Europe, central North America, Central America and Mexico, northeast Brazil, and southern Africa (IPCC 2012). The Maya Forest in the Mesoamerican region is experiencing below average rainfall and more intense droughts are predicted to occur in many areas due to climate change (Mardero et al. 2012). Drought poses an additional threat to mammals, especially those that are water dependent such as some ungulates (Terwilliger 1978; Naranjo 1995; Algers et al. 1998; Foerster and Vaughan 2002; Reyna-Hurtado et al. 2009).
The focal species of this study was Baird’s tapir (Tapirella bairdii) the largest land mammal in Central America. Historically, its geographic distribution extended from southern Veracruz, México, to northeastern Ecuador (Reid 2009; Garcia et al. 2016; Hernandez et al. 2020). Its elevational range extends from sea level to the mountains (3,620 masl; Naranjo 2009). Baird’s tapir exhibits an affinity for lowlands and it is highly dependent on water (Wainwright 2007). Tapirs play a unique role in forest regeneration due to their feeding ecology as a herbivore and seed disperser (Fragoso et al. 2003).
Central America has experienced a 70 % reduction of forest cover in the last 40 years, and Baird’s tapir has suffered a dramatic reduction in both distribution and population size over the past several decades. It is now estimated that Baird’s tapir populations are reduced in size by 50 % (Garcia et al. 2016). They were considered vulnerable by the International Union for the Conservation of Nature (IUCN) in 1996, but were subsequently uplisted to endangered in 2002 (IUCN 2013). Moreover, Baird’s tapir was ranked 34th in urgency for conservation among more than 4,000 mammal species assessed by experts from the Institute of Zoology of London due to its level of evolutionary distinctiveness and level of threat (Isaac et al. 2007). Tapirs were considered the 10th rarest forest mammal in the Neotropics (Dobson and Yu 1993). This alarming status is due to the combined effects of tapirs’ natural rarity, habitat loss, habitat fragmentation, overhunting, and their vulnerability to cattle-borne disease (Castellanos et al. 2008).
The Maya Forest, where the tapir thrives, is the largest continuous block of tropical forest in Mesoamerica and it is a site of global importance for biodiversity. It extends through parts of northwestern Belize, northern Guatemala, and southern Mexico. The Maya Forest is well known for its rich biodiversity, a substantial array of endemic species, and several ancient civilizations (Garcia-Gil and Pat 2001). This area is also highly important for the conservation of wide-ranging species that require extensive tracts of intact forest to sustain viable populations, including Baird’s tapir (Naranjo 2009) and the white-lipped peccary (Tayassu pecari; Reyna-Hurtado et al. 2009). The Maya Forest has been categorized as one of the strongholds for tapir populations due to its suitable habitats with large continuous areas that allow potential connectivity across the Yucatan Peninsula with northeastern Guatemala and Belize (Mendoza et al. 2013; Schank et al. 2015). Tobler (2002) showed that tapirs in southern Costa Rica were more abundant in areas with limited human presence. These requirements indicate that tapirs thrive in large and undisturbed protected areas as opposed to human dominated landscapes.
Despite tapirs’ level of extinction threat and their ecological and evolutionary relevance, there are critical gaps in our understanding of their basic ecology such as habitat use, habitat selection, spatial ecology and population demography (García et al. 2012). The availability of water resources in the Maya Forest has increasingly become indispensable for ungulates of concern such as Baird’s tapir (Reyna-Hurtado et al. 2019) and white-lipped peccary (Moreira-Ramirez et al. 2016). Due to the fact that water can be a limiting factor to Baird’s tapir populations (Krausman and Etchberger 1995; Cain et al. 2006; Krausman et al. 2006), and that the dry season (December to May) is becoming more intense and anthropogenic pressures (e. g., habitat fragmentation and road modernization) are increasing, we focused our study on assessing tapir occupancy and detection at waterholes at Runaway Creek Nature Reserve (RCNR) in Belize. Waterholes at this study site are depressions filled with water during the peak of the wet season.
Our primary objective was to estimate site-specific variables that influence the occurrence of tapirs by integrating the data into an occupancy analysis (MacKenzie et al. 2006). We used camera-trap data from waterholes at RCNR in Belize to test the hypothesis that the probability of tapir occupancy and detection were higher when further away from human infrastructure (e. g., villages, roads) and closer to topographic covariates (e.g., river, elevation).
Methods and Materials
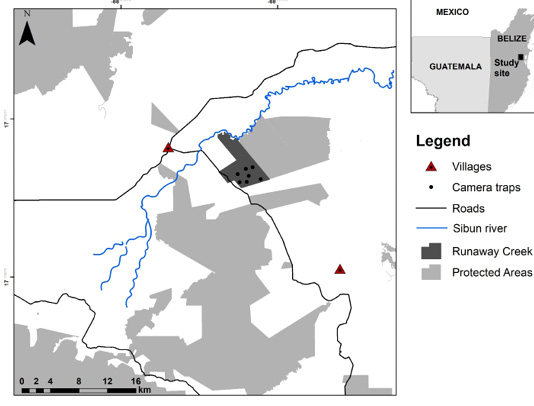
Study site. The Maya Forest is a tri-national and continuous tropical forest in Mesoamerica that encompasses the southern Mexican states of Campeche, Chiapas, Quintana Roo and Yucatan, the northern Petén department in Guatemala, and northwestern Belize. Our study site at Runaway Creek Nature Reserve (RCNR) is located in central Belize within the Belize district (Figure 1). Its location is at the southernmost region of the Maya Forest. RCNR is a core section of the Maya Forest Corridor within central Belize connecting the tri-national Maya Forest with the Chiquibul Maya Mountains massif of southern Belize. The corridor is considered the last viable region for wildlife species to persist. RCNR is a private nature reserve established in 1999 with an extension of 2,500 hectares at the geographical coordinates 17° 22’ N, -88° 35’ W. The elevation ranges from 20 to 120 m above sea level and the area has an annual precipitation of 2,000 to 2,200 mm. The region has two main seasons: wet (June to November) and dry (December to May). At the peak of the wet season, the river floods and supplies the seasonal waterholes with water and at the peak of dry season water becomes a limiting factor. Topographically, RCNR has a range of karst hills and falls within the watershed of the Sibun River. Vegetation types include pine savanna forest, broad leaf forest, riparian forest, and tropical lowland forest (Meerman 1999).
Methods. We conducted camera trap surveys at seven sites at RCNR. Camera-trap stations were deployed along trails leading to waterholes (aguadas). We selected waterholes based on their availability within the reserve and their capacity to retain water during the peak of the dry season. The average size of waterholes was 50 m2 and 2 m in depth at the peak of the wet season. The remote digital camera-traps (Bushnell 12MP Trophy camera HD, Brown) were attached to trees at a height of 40 to 50 cm above ground, and were set with a delayed trigger time of 0.8 seconds. The cameras were interspersed 1.0 to 3.7 km (mean = 2.5 km) apart to permit independence of capture events. This distance fits well with the most common estimate of Baird’s tapir home range of 1.0 to 3.0 km2 (Foerster and Vaughan 2002), but there are exceptions that demonstrate that tapirs could move up to 23 km2 (Reyna-Hurtado et al. 2016). Following Sandoval-Seres et al. (2016), we used a selection criterion of less than 24 hour intervals between camera capture events for considering independence. Camera-traps were checked every three weeks to download data and service the cameras.
Figure 1. Map depicts Runaway Creek Nature Reserve study site in Belize, showing the network of protected areas, camera trap site locations, roads, and villages.
We used the occupancy modeling methodology to infer the probability of tapir occupancy and detection with standard of error (SE) estimates. Occupancy is an alternative state variable to abundance, that uses the proportion when detection is lower than 1 (MacKenzie et al. 2006). We partitioned the detection history into 176 blocks of 7 days each and incorporated them in a single-season occupancy model using the program Presence 12.37 (Hines 2009). Although the data were collected over several seasons and years, we did not resample any sites in a multi-season framework. We do not believe that grouping the field seasons violates any of the assumptions of the modelling process because this analysis refers to occupancy as site use (Cove et al. 2013).
Seven candidate models were evaluated using five site-specific covariates. The covariates were selected based on known ecology of tapirs and possible factors that influence occurrence and detectability (Wainwright 2007; Padilla et al. 2010; Licona et al. 2011; Cove et al. 2013). We measured the nearest distances in km to three human disturbance areas or infrastructure (village, road, and forest edge) and two topographic factors (river and elevation) with the use of a Garmin GPS and Google Earth imagery (City and Country). All continuous covariate data were standardized to z scores for further analysis (MacKenzie et al. 2002) and the models were averaged using the maximum likelihood of occupancy (Burnham and Anderson 2002).
In this study we assumed that all survey sites had an equal probability of tapir detection (p) and occurrence (psi), so we allowed occupancy (Ѱ) to vary with the covariates. The models were evaluated using Akaike Information Criterion corrected for small sample size (AICc) and Akaike weights (w). All models considered were within 95 % CI (Confidence Intervals). The global model which includes all covariates was run to ensure that there were no covariate interactions. A Pearson correlation test was run on all covariates, and correlated covariates were not included in the analysis. The detection history together with the covariate were fitted in a single season occupancy model in PRESENCE 12.37 software (Hines 2009).
Results
The total sampling effort at RCNR was 8,932 camera-trap nights in two separate periods from March to December 2015, and January 2017 to October 2019, accumulating a total of 3.6 years of camera-trap sampling. No sampling was con ducted during the year 2016. The cumulative naïve occupancy estimate of both periods was 85.7 %; the probability of occupancy (psi) was 0.97 +/-0.15 (SE), and the probability of detection (p) was 0.14 +/- 0.01 for all sites and years. Using photo data from each camera site and the cumulative of all the years, the model that kept both occupancy and detection constant had the highest support with an AIC weight value of 0.49 (Table 1). However, models of distance to roads and villages had the highest influence in occupancy with AIC weight of 0.14 and 0.11, respectively. The river covariate was at the mid-section of models ranking with 0.09 of AIC weights. The global model and series of model combinations with road permutation and all covariates had the lowest support for occupancy model ranking.
Table 1 Occurrence probability models of Baird’s tapir based on camera traps at Runaway Creek Nature Reserve, Belize, showing the covariates with Akaike ranking and weights.
| Model | QAIC | Delta QAIC | AIC wgtModel | Likelihood | no.Par. | -2*LogLike |
|---|---|---|---|---|---|---|
| psi(.),p(.) | 488.18 | 0 | 0.49 | 1.00 | 2.00 | 726.27 |
| psi(Road),p(.) | 490.65 | 2.47 | 0.14 | 0.29 | 2.00 | 729.97 |
| psi(Village),p(.) | 491.18 | 3.00 | 0.11 | 0.22 | 2.00 | 730.77 |
| psi(River),p(.) | 491.41 | 3.23 | 0.09 | 0.19 | 2.00 | 731.12 |
| psi(Road and River),p(.) | 492.47 | 4.29 | 0.05 | 0.11 | 3.00 | 729.71 |
| psi(Road and village),p(.) | 492.56 | 4.38 | 0.05 | 0.11 | 3.00 | 729.84 |
| psi(Global),p(.) | 493.29 | 5.11 | 0.03 | 0.0777 | 6.00 | 721.93 |
The untransformed coefficient of covariates for occupancy models showed that villages (β = 0.77 +/- 0.99), and rivers (β = 0.47 +/- 0.86) had a positive relation, while roads (β = -0.95 +/- 0.87) had a negative relation for occupancy models (Table 2). Occupancy modeling was also conducted for each independent year with an increase of occupancy and detection probabilities from 2015 to 2019 (Table 3). Naïve occupancy estimate was 0.71, 0.51, 0.86, and 1.0, respectively for each year (Table 3).
Table 2 Model selection statistics and occurrence probabilities models with all covariates for Baird’s tapir based on camera traps at Runaway Creek Nature Reserve, Belize with untransformed coefficient.
| Untransformed coefficients of ovariates (SE)1 | |||||||
|---|---|---|---|---|---|---|---|
| Model | ∆2 | ω3 | K4 | (.)5 | Road | Village | River |
| Ѱ(.) p(.) | 0 | 0.32 | 2 | 3.32 (+4.47) | |||
| Ѱ(Road) p(.) | 2.47 | 0.09 | 2 | -0.95 (+0.87) | |||
| Ѱ(Village) p(.) | 3 | 0.07 | 2 | 0.77 (+0.99) | |||
| Ѱ(River) p(.) | 3.23 | 0.06 | 2 | 0.47 (+0.86) | |||
| Ѱ(Road and River) p(.) | 4.29 | 0.05 | 3 | -1.44 (+1.39) | -0.71 (+1.44) | ||
| Ѱ(Road and Village) p(.) | 4.38 | 0.05 | 3 | -0.85 (+0.93) | 0.38 (+1.05) | ||
1 Coefficients are in logit space and corresponds to each model covariate
2 AIC difference
3 Akaike weights
4 Number of model parameters
5 Model constant
Discussion
Our study of tapir occupancy at RCNR is unique because it is located at the core of the Maya Forest Corridor which is currently subject to the modernization of the Coastal road, and real estate development is rapidly encroaching on the last remaining forest in the area (Belize Press Office 2017). Despite the small sample size of camera sites, this study was reinforced with an extended period of camera trap sampling of the waterholes with 8,932 camera-trap nights over 3.6 years.
Table 3 Occurrence and detection probabilities rates with Standard Error (SE), Naïve occupancy estimates per year of Baird’s tapir based on camera traps at Runaway Creek Nature Reserve, Belize.
| Year | Occurrence probability | Detection probability | Naïve occupancy estimate |
|---|---|---|---|
| 2015 | 0.76 (0.18SE) | 0.13 (0.02SE) | 0.71 |
| 2017 | 0.57 (0.18SE) | 0.10 (0.02SE) | 0.51 |
| 2018 | 0.86 (0.13SE) | 0.16 (0.2SE) | 0.86 |
| 2019 | 1.00 (0SE) | 0.18 (0.02SE) | 1.00 |
This study represents the first attempt in Belize’s Maya Forest Corridor to evaluate the occurrence of Baird’s tapirs at waterholes using human infrastructure and topographic covariates. The rate of tapir occupancy appears to be stable at RCNR with a probability of occupancy (psi) of 0.97 +/- 0.15 and probability of detection (p) of 0.14 +/- 0.01 for all sites and years. Slight increases in occupancy and detections rates were seen from 2015 and 2019, which can be attributed to unusually extended dry seasons in central Belize (Meteorological Service of Belize 2020). This study is similar to a 11-year study conducted at another site in the Maya Forest, the Calakmul Biosphere Reserve in Mexico (Reyna-Hurtado et al. 2019), where tapir occupancy rate was relatively constant despite huge variations on water availability on the forest floor. However, caution should be taken when applying occupancy results as an alternate for tapir abundance. Sampling at waterholes tends to overestimate tapir abundance due to waterholes acting as lures for tapirs. A potential explanation could be that tapirs use waterholes more frequently in dry season due to the lack of water in the landscape (O’Farrill et al. 2014). Another reason for overall high occupancy probability at our study site could be related to tapir movement patterns. T. bairdii tends to reduce the size of their core activity areas during the dry season and remain near sources of water (Foerster and Vaughan 2002; Noss et al. 2003; Trolle et al. 2008). This cluster in tapir movement patterns near water sources probably increases the capture rate at cameras (Reyna-Hurtado et al. 2016). Nevertheless, our study results should serve as baseline information for further and systematic sampling of this rare and endangered species.
Human disturbance, especially villages, influenced tapir occurrence, as shown by our top ranked models where tapir occurrence increased with distance to human infrastructures. This finding concurred with Cove et al. (2013), who found that increasing distance from villages led to higher tapir abundance in Costa Rica, and with Tobler (2002), who also found that tapirs in southern Costa Rica were more abundant in areas with limited human presence. Interestingly, the covariate of roads had the highest ranking in the occupancy model but with a negative relation (β = -0.95 (0.87 SE)) with tapir occurrence. This implies that tapirs at RCNR are thriving despite nearby roads. A potential explanation for this phenomenon is the underdeveloped status of the Coastal road which is located on the southwestern boundaries of RCNR. The 60 km-long Coastal road connects the George Price Highway in central Belize to the Hummingbird Highway in the south, and it is unpaved with only two villages that each have a population of around 400 inhabitants. Hence, the Coastal road is practically isolated with very low traffic. On the contrary, the Burrell Boom Highway in central Belize is paved and has higher traffic flow. Poot and Clevenger (2018), documented a total of 14 tapir-vehicle collisions from 2008 to 2012 along the Burrell Boom Highway. This collision documentation corroborates our road model findings that tapirs thrive near roads, but with very alarming mortality results. Furthermore, observations from September 2018 to September 2019 of two radio collared tapirs present in the study site showed that both tapirs had an established home range only 20 m away from the Coastal road (unpublished data). Apparently, the nearest road at RCNR is not a deterrent for tapirs.
The topographic covariate river received little support from the occupancy models. However, observation on the two radio collared tapirs revealed an interesting behavior towards the river and waterholes. Baird’s tapir has recently been documented to have larger home ranges than previously reported. Jordan et al. (2019) documented a home range of 18.72 km2, Reyna-Hurtado et al. (2016) with 23.9 km2, and Naranjo (2009) documented a tapir moving 10 km to visit ponds. Observations on the radio collared tapirs at RCNR yielded a home range of 8 km2 and they did not use free flowing water bodies (river or creek) despite these being an average distance of 3.5 km away from the tapirs’ home ranges (unpublished data). Instead, they relied exclusively on waterholes for water requirements. These observations reinforce the importance of waterholes for tapirs at RCNR.
Our results suggest that Baird’s tapir occupancy probability rate at waterholes appears to be stable when further away from human infrastructure (villages) and thriving near the Coastal road. The topographic covariate (river) had minimal support. Apart from undisturbed areas being ideal habitat for tapirs, this study highlights: 1) the critical importance of waterholes to tapirs and thus the need for higher protection efforts of habitat with suitable waterholes which is necessary for the survival of tapirs, and 2) tapirs are thriving at close distance to undeveloped roads and the need for careful planning of the modernization of the Coastal road which may increase tapir vehicle collisions.
Conservation implications. This study reports the current status of Baird’s tapir on waterholes at RCNR. Apart from hunting, land use changes and increased infrastructure as main threats to the species, severe and prolonged dry seasons and their impact on the availability of water at ponds are also imminent threats (Magrin et al. 2007). More collaborative efforts on systematic research designs are essential not only for the conservation of tapir habitat but also for an understanding of waterhole usage dynamics. We propose a collaborative effort with the Belize Forest Department and Government of Belize in the installation of water containers on strategic waterholes like the Calakmul, Mexico model (Reyna-Hurtado et al. 2019). The continued monitoring of tapirs at waterholes on a long-term basis and with an increased sample size of waterholes and non-waterholes is also important. This initiative will identify which waterholes are of more importance to tapirs, and determine the rate of abundance, as well as find trends of usage by tapirs for management and conservation purposes. In addition, it is imperative to conduct more studies on movement ecology of tapirs. This type of study will shed light on the usage dynamics and movement patterns among the network of waterholes and within the Maya Forest Corridor. This study sets the path for future and similar studies in the Maya Forest where strategic research is needed to enhance survival and viability of the Baird’s tapir population which is estimated to include 1,000 to 1,500 individuals in the region (Garcia et al. 2016).











 nueva página del texto (beta)
nueva página del texto (beta)