Introduction
The systematic study of daily habits of animals began in 1920 with the work of Szymanski (Halle and Stenseth 2000). Since then, several studies have been conducted on various animal species, mainly in captivity or laboratory conditions (DeCoursey 1990). Photographic cameras were first used by Pearson (1959, 1960) to monitor activity patterns of the California vole (Microtus californicus) in the late 1950s. Camera traps have allowed studying the temporal behavior of animals in natural and captivity conditions (e. g., Kachamakova and Zlatanova 2014). The development of more efficient camera traps has now allowed a better approach to the study of activity patterns (Oliveira-Santos et al. 2008). The use of camera traps provides a noninvasive research method that, with relatively little effort, yields information on multiple species, including those that are rare, elusive, or little known (Sanderson and Trolle 2005; Jimenez et al. 2010; O’Brien and Kinnaird 2011). In addition to their use for recording and documenting species presence, camera traps are also useful for recording their behavior, thus supporting the conduct of community-level analyses (Rowcliffe and Carbone 2008; Whitworth et al. 2016).
According to Halle and Stenseth (2000), studies on the temporal behavior of animals can be classified into two fields: 1) activity patterns, in which the activities of animals are examined during two periods of time: the active period, when animals perform activities such as foraging, defense of territory, exploration, and others; and the rest period, when animals carry out “comfort behavior”, including sleeping, grooming, social contact, brood care, among others, and 2) time allocated to the various activities carried out by animals during the day.
Much of the knowledge on the activity patterns of mammals has come from studies on terrestrial species at ground level (e. g.,Van Schaik and Griffiths 1996; Gómez et al. 2005; Gliwicz and Dabrowski 2007; Chen et al. 2009), whereas arboreal mammal species have been scarcely studied, mainly due to the technical challenges involved in observing them in the forest canopy. However, the development of technology and affordable sampling techniques have made it possible to make progress in the study of the ecology of the forest canopy fauna (e. g.,Charles-Dominique 1975; Van Schaik and Griffiths 1996; Schipper 2007; Bowler et al. 2017; Whitworth et al. 2016).
The description of activity patterns initially targeted a few rodent species including, for example, the red squirrel (Sciurus vulgaris) in coniferous forests and the Panamanian climbing rat (Tylomys panamensis) in tropical ecosystems (Wauters 2000; Mendez-Carvajal et al. 2015). Fernández-Duque (2003) documented the activity pattern of the Azara’s night monkey (Aotus azarae) in the western slope of South America, while Schipper (2007) monitored the kinkajou (Potos flavus) in Costa Rica. In recent years, similar studies have been carried out in tropical ecosystems around the world, particularly in Latin America, documenting the species richness of forest canopy mammals (Whitworth et al. 2016). One of the earliest studies on forest canopy mammals that adopted a community-wide perspective was conducted in Brazil (Oliveira-Santos et al. 2008), in which the authors were able to document the presence of at least 11 mammal species in five families. The use of camera traps as a means to gather data on various species has become common in Mexico (Mandujano 2019). However, studies focusing on the community of forest canopy mammals in the Mexican tropics have not yet been carried out or published. For this reason, gathering ecological information on forest canopy mammals is now essential. This study aimed to document the species richness of mammals inhabiting the tree stratum, describing their activity patterns, and evaluating the temporal overlap between them in a tropical forest on the Gulf of Mexico.
Materials and Methods
The study was conducted in the Santa Gertrudis Ecological Reserve (RESG, for its acronym in Spanish), located in the Municipality of Vega de Alatorre, State of Veracruz, Mexico (19º 51’ 49.22” to 19º 33’ 35.25” N and -96º 32’ 27.49” to -96º 36’ 52.41” W; Figure 1). The RESG is a 1,000-ha private property dedicated to nature conservation and decreed as a federal Zone for Forest and Fauna Protection (SARH 1982). The dominant vegetation is a semideciduous tropical forest in different successional stages located at the northernmost limit of the distribution range of the humid tropical forest in Mexico. The study area also includes riparian forests (Godínez-Ibarra and Lopez-Mata 2002), old coffee plantations, remnants of mountain cloud forests, and pastures (B. Vega Hernandez pers. comm). Elevation in the study area ranges between 80 and 940 masl and temperature, between 18.6 and 25.7 °C; the mean annual precipitation is 1,605 mm (CONAGUA 2016).
Data were collected employing camera traps over 12 months, from mid-February 2016 until mid-February 2017. Nine camera traps fitted with infrared motion sensors (Ltl. Acorn, Wild view Tk40, and Cuddeback C2) were installed. The camera traps were placed in the tree canopy (~8 to 12 m above the ground), affixed to the trunk; these cameras were set focusing on a branch overlapping a neighboring tree. These interconnections between trees are known as “canopy highways” that allow mammals to move between trees and increase their probability of detection (Schipper 2007). We used tree climbing gear and techniques to reach the tree canopy. Camera traps were left operating during the entire sampling period and were checked monthly to retrieve the images captured and replace the batteries. The cameras were set to shoot three photographs per event at 1-min intervals.
All consecutive photographs of different species, as well as all photographs of the same species separated by at least one hour, were regarded as independent records. The distance between cameras ranged from 300 to 1,500 m, depending on the terrain accessibility and availability of trees suitable for affixing the cameras. The trees selected had a diameter at breast height (DBH) ≥ 40 cm and at least one branch overlapping those of other trees. The mammals recorded were identified based on specialized guides and literature (e. g., Emmons and Feer 1997; Reid 1997, 2004).
Activity patterns were elucidated using the software Oriana 4.0 (Kovach 2011). As recommended by Ridout and Linkie (2009), only those species with at least ten independent captures were included in these analyses. The times of capture were considered as a random sample taken at any time of the day. The activity pattern of each species was classified based on the hourly records. Data were grouped according to the classification proposed by Van Schaik and Griffiths (1996), as modified by Gómez et al. (2005). We identified four periods of activity: 1) diurnal, species with ≥ 70 % of captures during the daytime, 2) nocturnal, species with ≥ 70 % of captures during the night-time, 3) crepuscular, 50-60% of captures during twilight (the period one hour before and one hour after dawn or dusk), and 4) cathemeral, species with captures that showed no distinctive pattern or were randomly distributed along the daily cycle.
We used the package Overlap for R 3.4.1 (R Core Team 2014) to compare the activity patterns and evaluate the overlap between species. The coefficient of overlapping, Δ, is defined as the area under the curve formed by taking the minimum of the two probability density functions at each time point (Ridout and Linkie 2009). The estimator Δ1 is recommended for analyzing activity patterns when the number of records is less than 25 (Ridout and Linkie 2009). Coefficient values range from 0 to 1, with 0 indicating that the activities of species do not coincide at any time of the day, and ~1 or 1 that their times of activity are the same throughout the day (Ridout and Linkie 2009). We used the bootstrap method with 500 samples to construct 95 % confidence intervals for Δ1 (Lynam et al. 2013).
Results
We invested a total sampling effort of 2,664 camera-nights and collected 176 records of 12 species in seven families and four orders. All the species recorded showed a diurnal or nocturnal activity pattern; no species with crepuscular or cathemeral patterns were registered (Table 1).
Species Records and Abundance. The species most frequently recorded are common in tropical ecosystems (Table 1). However, we also recorded some species that are particularly important by their conservation status; these include the Mexican tree porcupine (Coendou mexicanus) and the margay (Leopardus wiedii), each with seven records, and the kinkajou (P. flavus) with 25records, the latter being one of the species most frequently recorded. It is worth noting that we documented two arboreal rodents: Peters’s climbing rat (Tylomys nudicaudus), with 20 records, and the vesper rat (Nyctomys sumichrasti), with only one register. Our results show that the most abundant species was the Mexican gray squirrel (Sciurus aureogaster) with 53 records; the least abundant ones were two species in the family Didelphidae (Marmosa mexicana and Philander opossum) with one register each.
Table 1 Species recorded, common name, number of independent records, abundance index, period of activity, and activity pattern.
| Orden | Specie | Common name | Number of records | Abundance index | Time of activity | Activity pattern |
|---|---|---|---|---|---|---|
| Didelphimorphia | Marmosa mexicana | Mouse opossum or Tlacuatzin | 1 | 0.375 | Nocturnal | 0:15 |
| Didelphis marsupialis | Opposum or Tlacuache | 41 | 15.39 | Nocturnal | 19:00-5:40 | |
| Philander opossum | Four Eyes Opposum or Tlacuache de cuatro ojos | 1 | 0.375 | Nocturnal | 22:00 | |
| Pilosa | Tamandua mexicana | Anteater or Oso hormiguero | 11 | 4.129 | Nocturnal | 21:50-5:02 |
| Rodentia | Nyctomys sumichrasti | Arboreal rodent or Ratón | 1 | 0.375 | Nocturnal | 0:25 |
| Tylomys nudicaudus | Arboreal Rat or Rata | 20 | 7.507 | Nocturnal | 20:30-5:10 | |
| Coendou mexicanus | Porcupine or Puercoespín | 7 | 2.627 | Nocturnal | 20:00-5:00 | |
| Sciurus aureogaster | Squirrel or Ardilla gris | 53 | 19.894 | Diurnal | 6:40-18:40 | |
| Carnívora | Leopardus wiedii | Margay or Tigrillo | 6 | 2.252 | Nocturnal | 20:10-4:44 |
| Bassariscus sumichrasti | Ringtail or Sietillo or huiloncha | 2 | 0.75 | Nocturnal | 21:46-1:00 | |
| Nasua narica | Tejón o Coatí | 8 | 3.003 | Diurnal | 08:50-21:50 | |
| Potos flavus | Kinkajou or Martucha | 25 | 9.384 | Nocturnal | 20:30-4:50 |
Daily Activity of Each Species. Only five of the 12 species recorded were included in the activity pattern analysis, due to the insufficient number (< 10) of independent records obtained for the remaining seven species (Table 1). Only one species, S. aureogaster, showed a diurnal pattern. The remaining four species were classified as nocturnal; their activity period comprised between 19:00 h and 5:00 h (Figure 2).
The activity of the four nocturnal species peaked between 22:00 h and 2:00 h and dropped towards dawn; they showed similar activity peaks. It should be noted that the activity period of the common opossum (Didelphis marsupialis) was more extended than those of the other species (Figure 2) and showed two activity peaks at both ends of the period: around 22:00 h and 4:00 h.
Overlap of Activity Patterns Only the four nocturnal species were included in the analysis of the overlap of daily activity patterns. S. aureogaster was excluded because its activity pattern was entirely diurnal, with no overlap with the pattern of other species.
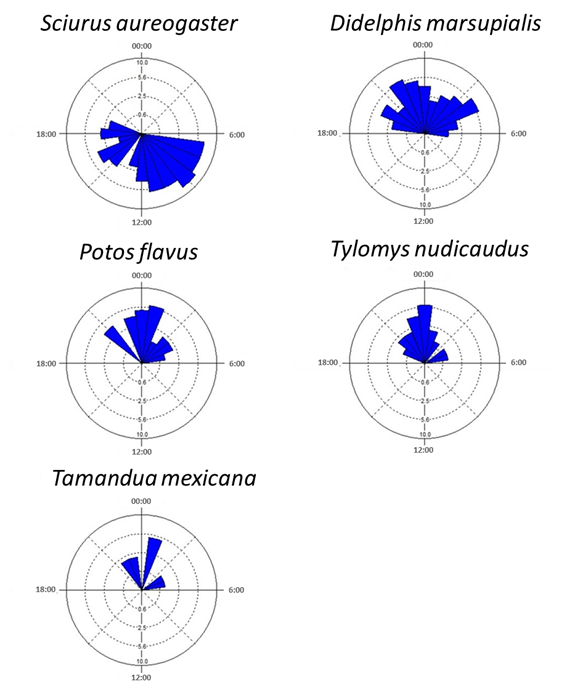
Figure 2 Daily activity patterns of canopy mammals. Blue bars denote the number of records on each hour of the day.
The activity patterns of the four species examined showed a relatively high level of overlap; the overlapping coefficients, Δ1, ranged from 0.696 to 0.919 (Figure 3). The estimated overall average overlapping of the combinations of the four species is close to 1 (Δ1 = 0.801 ± 0.065 D. E.). D. marsupialis and T. nudicaudus were the species for which activity patterns showed the lowest overlap; the greatest overlap was found between T. mexicana and P. flavus (Figure 3).
Discussion
We recorded 12 of the 14 arboreal mammal species that have been reported for this region (González-Christen and Delfin-Alfonso 2016). Four of these species are listed in an extinction-risk category as the NOM-059-SEMARNAT-2010 (Diario Oficial de la Federación 2010, vr. 2019): the tropical cacomixtle or ringtail cat (Bassariscus sumichrasti) and the kinkajou (Potos flavus) are listed as under special protection; the Mexican tree porcupine (Coendou mexicanus), as threatened; and the margay (Leopardus wiedii), as endangered. Species not recorded in our study were the raccoon (Procyon lotor) and the tayra (Eira barbara). Gallina and González-Romero (2018) evaluated the diversity of terrestrial mammals in the RESG and recorded only nine species, including P. lotor, but did not record E. barbara either.
The Mexican gray squirrel (S. aureogaster) was active during the daylight period, a result consistent with that reported by Ramos-Lara and López-González (2017) for a pine-oak forest in the Sierra Gorda Biosphere Reserve (RBSG). A similar activity pattern was recorded for the gray squirrel in our study and the one by Ramos-Lara and López-González (2017), although a peak was recorded around 09:00 h in the RBSG, continuous activity was observed throughout the day, with no evident peak, in the RESG. This difference might be because S. aureogaster coexists with S. oculatus in the RBDG, which might lead to niche segregation. By contrast, no other squirrel that might compete for resources with S. aureogaster occurs in the RESG.
The observed activity pattern of P. flavus (see Figure 2) is consistent with the one documented in French Guyana (Julien-Laferriere 1993), although the activity peaks also differed. Our results show a unimodal activity pattern in the RESG, whereas it is bimodal in French Guyana (Julien-Laferriere 1993). This difference in activity peaks may be due to two reasons: 1) difference in sampling techniques, as Julien-Laferriere used telemetry to monitor three individuals for 12 days, which allowed him to track their activity 24 hours a day, and 2) difference in the ecological characteristics of the habitat (e. g., climate, the structure of the tropical forest, food availability, presence of predators, among others) that might influence the activity patterns of P. flavus.
Our results show that the anteater (T. mexicana) had a nocturnal activity pattern (21:50-05:02 h); this is entirely different from what has been reported by other studies in tropical and subtropical ecosystems. For example, Brown (2011) concluded that the activity pattern of this species in Barro Colorado Island, Panama, can be considered quasi cathemeral with a tendency to diurnal (7:00 - 22:00 h) and a peak around 15:00 h. This species has been described as having highly variable habits, as they can be active during the daytime, the nighttime, or both (Montgomery 1985 a, b). The fact that the anteater displays a nocturnal activity pattern in some sites (as in this case) and diurnal in others evidences its ability to adapt to different or unfavorable situations (Montgomery 1985a, b; Brown 2011). One factor that might influence the observed activity pattern is the evasion of predators such as felines, raptors, or snakes (Izor 1985; Aranda 1994), or even poachers who often enter illegally the RESG with dogs (B. Vega Hernandez pers. comm.).
One factor that often limits the study of arboreal mammals is the low register success; a three-dimensional space such as the forest canopy makes detection difficult, as mammals can move along any of the three dimensions of the canopy. This is in clear contrast with ground-level studies in which only two dimensions are relevant, showing capture-success rates usually over 15 % (Tobler et al. 2008; Lira-Torres and Briones-Salas 2012). Gallina and González-Romero (2018) attained a 14.5 % capture success in the RESG.
Our study is a first effort to document the species richness and activity patterns of mammals in the canopy of a tropical forest in Mexico. We recognize the importance of observing and describing in detail the activity patterns of mammals inhabiting tropical and subtropical ecosystems, although we were somewhat limited in the number of records obtained for some species. In this regard, we suggest carrying out longer-term studies to better understand how assemblages of arboreal mammals are distributed in space and time, allowing species coexistence.











 nova página do texto(beta)
nova página do texto(beta)




