Introduction
With ca. 2600 species, rodents compose the most diverse order within Mammalia, including about 42 % of the living mammal species (D’Elía et al. 2019a). Native rodents inhabit almost every habitat on Earth, except Antarctica, New Zealand, and some oceanic islands. They occupy a large variety of terrestrial and freshwater niches, including even gliding species (D’Elía et al. 2019a). As other small mammals, rodents play a fundamental role in trophic chains, acting as preys of other vertebrates, and contributing to energy and nutrient flow, providing key functions to the ecosystems, such as seed dispersal or soil removal (Lacher et al. 2017). Despite these characteristics, rodents are among the least known mammals, both in terms of their taxonomy and natural history, with many species that are known only from the holotype or type series and frequently from collections made more than a century ago (Amori et al. 2016).
From a conservational perspective, even when laudable efforts exists (notable the IUCN Small Mammal Specialist Group, which also covers tree shrews and eulipotyphlans), rodents are not charismatic species as other medium and large mammals (e. g., canids, cetaceans, felids or ungulates), attracting little attention from media and financial founds (Fleming and Bateman 2016). This is an unfortunate situation, since the historical record indicates that rodents are among the most vulnerable mammals to extinction owing to direct or indirect human activities, accounting ca. 53 % of the total number of mammal losses during the last 500 years (Turvey 2009).
As other small mammals, several species of rodents have restricted geographical ranges, a fact that could increase their immediate risk of extinction (e. g., Gaston 1994). It is widely accepted that species having restricted distributions and/or low population sizes have usually lower genetic variation than wide-ranging relatives (e. g., Caughley 1994; MacArthur and Wilson 1967), being highly susceptive to disruptive treats, such as new or introduced competitors, pathogens, and predators, severe climatic events (e. g., droughts), cataclysms (e. g., volcanic eruptions), and/or population-level phenomena (e. g., inbreeding depression). An eloquent example of the vulnerability of rodents with restricted distributional ranges is the biological extinctions of ca. 21 endemic species from the Caribbean islands since 1500 AD after the European colonization (Turvey 2009).
At the time of description, new species are known at least from one locality (i. e., the type locality) and a single individual (i. e., the holotype), on which the species description is based. The known distribution of most species is normally enlarged as new specimens are recorded in other localities. However, some species remaining being known from only the vicinities of their type localities; sometimes, this fact reflects the existence of microendemisms (e. g., Phyllotis bonariensis, which is apparently restricted to the hilly system of Ventania in central-eastern Argentina; see Steppan and Ramírez 2015), but in most cases is only because of the limited field work or to already collected specimens have not been identified as representative of those species.
In this contribution, we reviewed the distribution patterns, main habitats, use of substrate, and conservation implications of South American rodents that are only known from their type locality or its immediate surroundings. We also discussed if these species are geographically distributed in poorly surveyed areas or if they share some life traits that may make them easy to overlook (e. g., fossoriality).
Materials and methods
To identify those species only known from their type localities and/or its immediate surroundings, we reviewed the most recent compilations on Neotropical rodents, using Patton et al. (2015) as starting point. For those species described since 2015, we consulted the review of D’Elía et al. (2019a) plus the literature published after December 2017, which is the date that ends the period included in this review. We also reviewed the primary literature. In each case, we individually reviewed the distributional range of each taxon, searching in published (e. g., Patton et al. 2015) and online data sources (e. g., GBIF, http://www.gbif.org). Taxonomy follows Patton et al. (2015), with minor modifications according to the posterior literature.
We use the definition of type locality given by Meiri et al. (2017), which considered a maximum latitudinal and longitudinal range of < 10 km (= 0.1º). This restriction is in accordance with an extent of occurrence < 100 km2, which fits partially with the criterion B1 of the IUCN for an extent of occurrence of a critically endangered species (IUCN, 2017).
For each species, we distinguished between those known only from old records and those recently described or known by repeated records in the type locality. The cut-off between old and recent records was arbitrarily placed at 50 years ago (1969; see Meiri et al. 2017 for a similar procedure).
Use of substrate for each species was taken from the literature (e.g., Patton et al. 2015). Six main habitat categories were considered in the analysis, following the proposal of Amori et al. (2016): i) deserts, ii) grasslands, iii) scrublands, iv) temperate forests, v) tropical forest, vi) unknown.
Results
We identified 58 species of South American rodents that are known only from their type localities or their vicinities (Figure 1, Table 1). These species belong to seven families, of which six belong to Hystricomopha (Table 1). However, the family with more species (n = 28; 48.3 % of the total) in this list is Cricetidae, all belonging to the subfamily Sigmodontinae. The 58 identified species are part of 29 genera; the genus with most species in the list is the ctenomyid Ctenomys with 11, followed by the cricetid Thomasomys with five. Remarkably, some of the South American rodent species known only from the surroundings of their type localities are relatively large animals as the mountain vizcacha Lagidium ahuacaense (2,000 g), recorded at a single rocky outcrop point in the coastal Desert of Ecuador and the chinchilla rat Cuscomys ashaninka (910 g) only know from its holotype collected at a Peruvian humid cloud forest (Emmons 1999; Spotorno and Patton 2015).
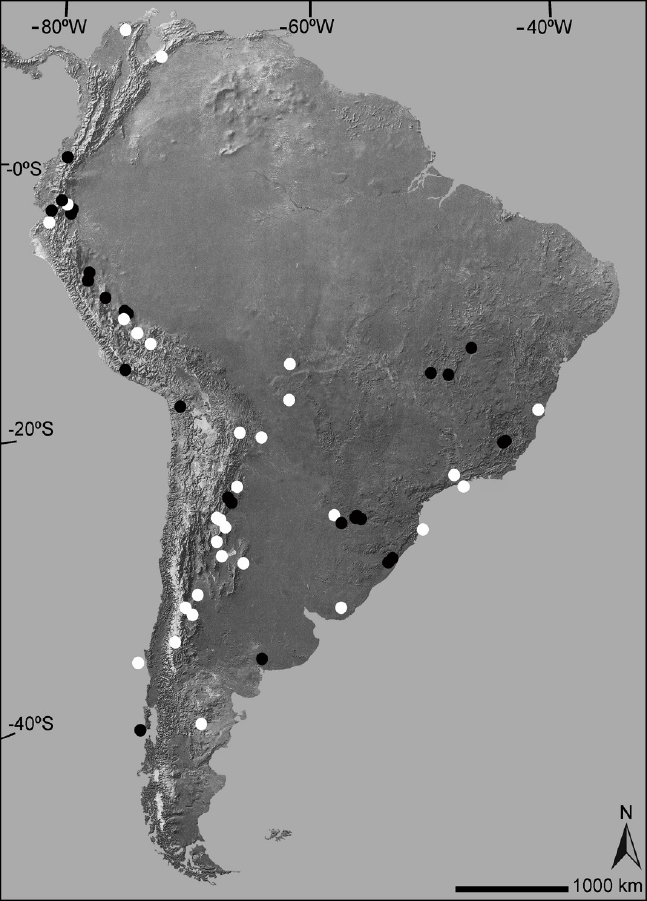
Figure 1 Map of South America depicting the geographical distribution of those rodent species only known from their type localities (black circles = sigmodontine; white circles = caviomorph).
Table 1 List of species of South American rodent that are only known from their type localities.
| Suborder | Family | Year of description | Habitat | Country | Habits | UICN | |
| Akodon kotosh | Supramyomorpha | Cricetidae | 2016 | tropical forest | Peru | cursorial | NE |
| Akodon mystax | Supramyomorpha | Cricetidae | 1998 | grassland | Brazil | cursorial | DD |
| Akodon philipmyersi | Supramyomorpha | Cricetidae | 2005 | grassland | Argentina | cursorial | DD |
| Brucepattersonius guarani | Supramyomorpha | Cricetidae | 2000 | tropical forest | Argentina | cursorial | DD |
| Brucepattersonius misionalis | Supramyomorpha | Cricetidae | 2000 | tropical forest | Argentina | cursorial | DD |
| Brucepattersonius paradisus | Supramyomorpha | Cricetidae | 2000 | tropical forest | Argentina | cursorial | DD |
| Calomys chinchilico | Supramyomorpha | Cricetidae | 2007 | desert | Peru | cursorial | NE |
| Cerradomys akroai | Supramyomorpha | Cricetidae | 2014 | scrubland | Brazil | cursorial | NE |
| Deltamys araucaria | Supramyomorpha | Cricetidae | 2017 | tropical forest | Brazil | cursorial | NE |
| Euneomys fossor | Supramyomorpha | Cricetidae | 1899 | uncknown | Argentina | uncknown | DD |
| Geoxus lafkenche | Supramyomorpha | Cricetidae | 2016 | temperate forest | Chile | fossorial | NE |
| Hylaeamys tatei | Supramyomorpha | Cricetidae | 1998 | tropical forest | Ecuador | cursorial | DD |
| Juliomys ximenezi | Supramyomorpha | Cricetidae | 2016 | tropical forest | Brazil | climber | NE |
| Juscelinomys candango | Supramyomorpha | Cricetidae | 1965 | scrubland | Brazil | cursorial | EX |
| Microakodontomys transitorius | Supramyomorpha | Cricetidae | 1993 | scrubland | Brazil | cursorial | EN |
| Neacomys macedoruizi | Supramyomorpha | Cricetidae | 2018 | tropical forest | Peru | cursorial | NE |
| Necromys lilloi | Supramyomorpha | Cricetidae | 2016 | grassland | Argentina | cursorial | NE |
| Nectomys saturatus | Supramyomorpha | Cricetidae | 1897 | tropical forest | Ecuador | semiaquatic | NE |
| Oxymycterus caparaoe | Supramyomorpha | Cricetidae | 1998 | grassland | Brazil | cursorial | LC |
| Phyllotis anitae | Supramyomorpha | Cricetidae | 2007 | grassland | Argentina | cursorial | DD |
| Phyllotis bonariensis | Supramyomorpha | Cricetidae | 1964 | grassland | Argentina | cursorial | NT |
| Phyllotis osgoodi | Supramyomorpha | Cricetidae | 1950 | desert | Chile | cursorial | DD |
| Rhipidomys albujai | Supramyomorpha | Cricetidae | 2017 | tropical forest | Ecuador | climber | DD |
| Thomasomys apeco | Supramyomorpha | Cricetidae | 1993 | tropical forest | Peru | cursorial | VU |
| Thomasomys fumeus | Supramyomorpha | Cricetidae | 1924 | tropical forest | Ecuador | cursorial | DD |
| Thomasomys hudsoni | Supramyomorpha | Cricetidae | 1923 | scrubland | Ecuador | cursorial | VU |
| Thomasomys onkiro | Supramyomorpha | Cricetidae | 2002 | tropical forest | Peru | cursorial | VU |
| Thomasomys rosalinda | Supramyomorpha | Cricetidae | 1926 | tropical forest | Peru | cursorial | EN |
| Abrocoma budini | Hystricomorpha | Abrocomidae | 1920 | scrubland | Argentina | cursorial | DD |
| Abrocoma famatina | Hystricomorpha | Abrocomidae | 1920 | scrubland | Argentina | cursorial | DD |
| Abrocoma vaccarum | Hystricomorpha | Abrocomidae | 1921 | scrubland | Argentina | cursorial | DD |
| Cuscomys ashaninka | Hystricomorpha | Abrocomidae | 1999 | tropical forest | Peru | cursorial | DD |
| Cuscomys oblativus | Hystricomorpha | Abrocomidae | 1916 | tropical forest | Peru | cursorial | DD |
| Cavia intermedia | Hystricomorpha | Caviidae | 1998 | grassland | Brazil | climber | CR |
| Cavia patzeli | Hystricomorpha | Caviidae | 1981 | grassland | Ecuador | climber | DD |
| Lagidium ahuacaense | Hystricomorpha | Chinchillidae | 2009 | desert | Ecuador | cursorial | DD |
| Lagostomus crassus | Hystricomorpha | Chinchillidae | 1910 | grassland | Peru | cursorial | EX |
| Ctenomys andersoni | Hystricomorpha | Ctenomyidae | 2014 | scrubland | Bolivia | fossorial | NE |
| Ctenomys bicolor | Hystricomorpha | Ctenomyidae | 1914 | scrubland | Bolivia | fossorial | NE |
| Ctenomys brasiliensis | Hystricomorpha | Ctenomyidae | 1826 | grassland | Uruguay | fossorial | DD |
| Ctenomys fochi | Hystricomorpha | Ctenomyidae | 1919 | grassland | Argentina | fossorial | DD |
| Ctenomys johanis | Hystricomorpha | Ctenomyidae | 1921 | scrubland | Argentina | fossorial | DD |
| Ctenomys juris | Hystricomorpha | Ctenomyidae | 1920 | scrubland | Argentina | fossorial | DD |
| Ctenomys lessai | Hystricomorpha | Ctenomyidae | 2014 | grassland | Bolivia | fossorial | NE |
| Ctenomys osvaldoreigi | Hystricomorpha | Ctenomyidae | 1995 | grassland | Argentina | fossorial | CR |
| Ctenomys paraguayensis | Hystricomorpha | Ctenomyidae | 2000 | grassland | Paraguay | fossorial | NE |
| Ctenomys pontifex | Hystricomorpha | Ctenomyidae | 1918 | scrubland | Argentina | fossorial | DD |
| Ctenomys validus | Hystricomorpha | Ctenomyidae | 1977 | scrubland | Argentina | fossorial | DD |
| Ctenomys yatesi | Hystricomorpha | Ctenomyidae | 2014 | scrubland | Bolivia | fossorial | NE |
| Ollalamys edax | Hystricomorpha | Echimyidae | 1916 | tropical forest | Venezuela | climber | DD |
| Phyllomys mantiqueirensis | Hystricomorpha | Echimyidae | 2003 | tropical forest | Brazil | climber | CR |
| Phyllomys thomasi | Hystricomorpha | Echimyidae | 1897 | tropical forest | Brazil | climber | EN |
| Phyllomys unicolor | Hystricomorpha | Echimyidae | 1842 | tropical forest | Brazil | climber | CR |
| Octodon pacificus | Hystricomorpha | Octodontidae | 1994 | temperate forest | Chile | cursorial | CR |
| Tympanoctomys aureus | Hystricomorpha | Octodontidae | 2000 | desert | Argentina | fossorial | CR |
| Tympanoctomys kirchnerorum | Hystricomorpha | Octodontidae | 2014 | desert | Argentina | fossorial | DD |
| Tympanoctomys loschalchalerosorum | Hystricomorpha | Octodontidae | 2000 | desert | Argentina | fossorial | CR |
| Santamartamys rufodorsalis | Hystricomorpha | Octodontidae | 1899 | tropical forest | Colombia | climber | CR |
Most species included in our list were described during the decades of 1890 to 1930 (n = 18; 31 %) and 1990 to recent (n = 33; 56.9%), with a peak between 1990-2000 (n = 14; 24.1 %); Table 1; Figure 2). More than the half of the surveyed species (n = 36; 61.1 %) were described since 1969. At least four species of those described prior to 1969 (i. e., Ctenomys bicolor, Phyllomys thomasi, Phyllotis bonariensis, and Santamartamys rufodorsalis) were recorded again from their type localities during the last 50 years (cf. Patton et al. 2015).
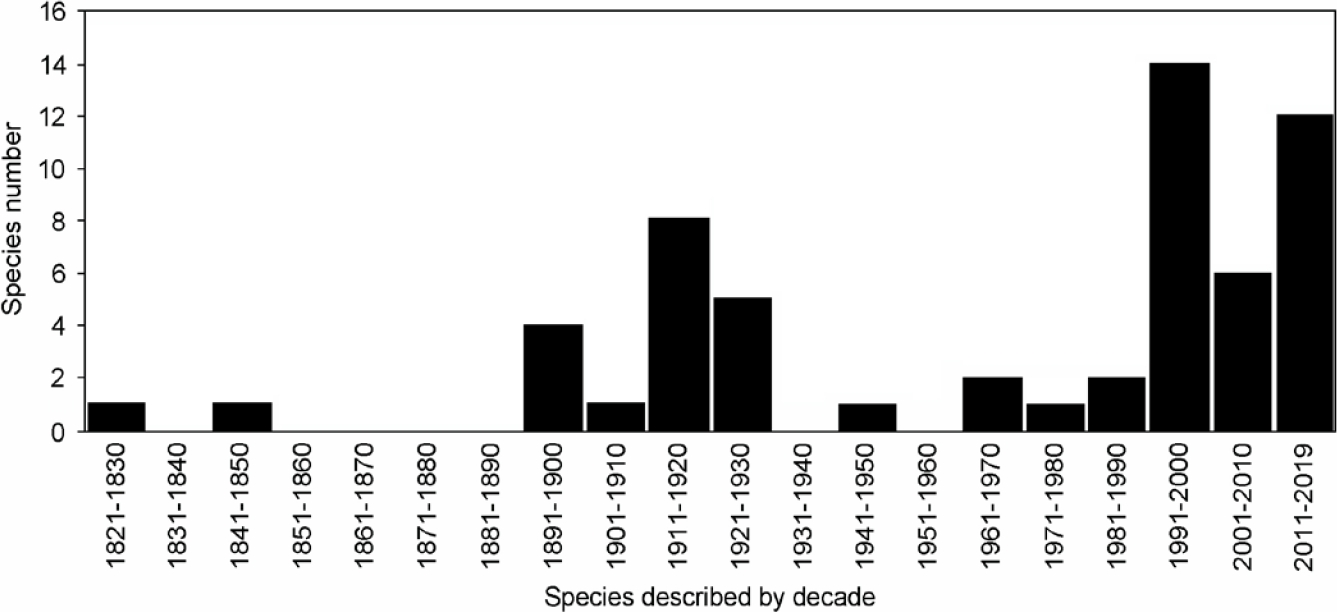
Figure 2 Number of South American rodent species described by decade between 1820 and the present that are only known from their type localities.
The majority of the selected species were collected at open to brushy and arid-semiarid to temperate habitats, including deserts (n = 6; 10.3 %), grasslands (n = 14; 24.1 %), and scrublands (n = 14; 24.1 %). However, the habitat type with more species in the list was tropical forest (n = 21; 36.2 %; Table 1; Figure 3). Looking at the country of origin, we observed that a high number of the species in our list occurs in Argentina (n = 21; 36.2 %), Brazil (n = 12; 20.7 %), and Peru (n = 9; 15.5 %). Other seven countries are represented by 1 (Colombia, Paraguay, Uruguay, Venezuela) to 3 (Bolivia) or 6 (Ecuador) species (Table 1). No species comes from Guyana, Suriname or French Guiana.
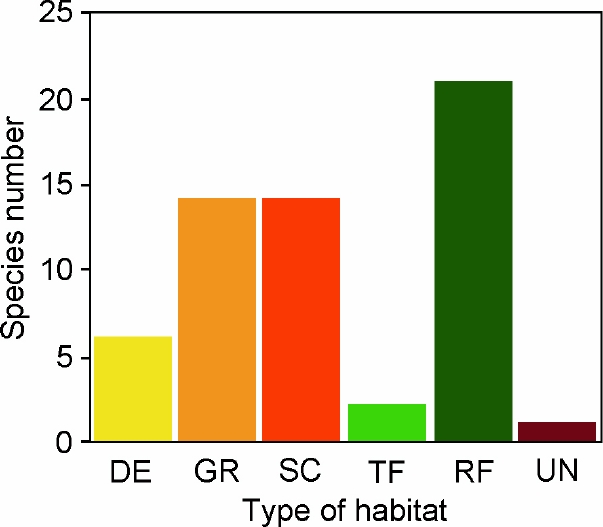
Figure 3 Number of South American rodent species by habitat type that are only known from their type localities. Abbreviations are as follow: deserts (DE); grasslands (GR); scrublands (SC); temperate forests (TF); tropical forest (RF); unknown (UN).
Regarding the use of substrate, four major groups can be recognized: cursorial (n = 32; 55.2 %), fossorial (n = 16; 27.6 %), climber (n = 9; 15.5 %), and semiaquatic (n = 2; 3.4 %; Table 1).
In terms of conservation status as considered by the IUCN Red List, two species (3.4 %) are considered as extinct (EX), including the akodont Juscelinomys candango and the vizcacha Lagostomus crassus (Table 1; Figure 4). Almost a third of the remaining species are considered under the highest categories of threat, such as critically endangered (CR; n = 8; 13.8 %), endangered (EN; n = 3; 5.2 %), vulnerable (VU; n = 3; 5.2 %) or near threatened (NT; n = 1; 1.7 %). Only one species is considered as of least concern (LC; n = 1; 1.7 %); while almost half of the species in our list are referred as data deficient (DD; n = 26; 44.8 %). Finally, the IUCN has not yet evaluated most of the species described or removed from the synonymy of other taxa since 2014; consequently these species are listed as not evaluated (NE; n = 14; 24.1 %; Table 1; Figure 3).
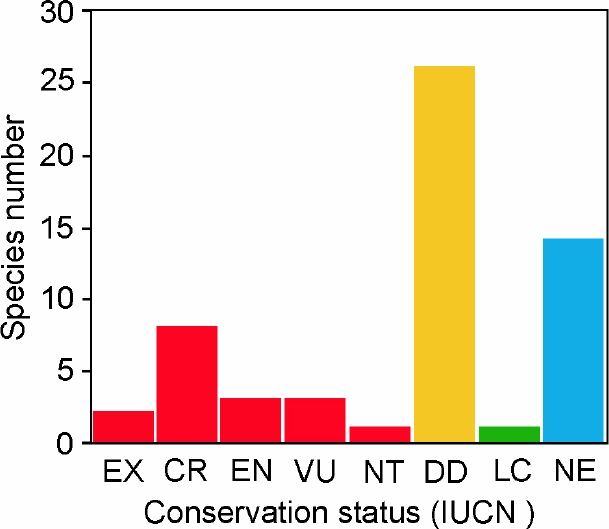
Figure 4 Number of South American rodent species according to their category in the IUCN’s Red List that are only known from their type localities. Abbreviations are as follow: EX, extinct; CR, critically endangered; EN, endangered; VU, vulnerable; NT, near treathened; LC, least concern; DD, data deficient, NE, not evaluated
Discussion
Our study document that 58 rodent species from South American are only known from their type localities and their surroundings; i. e., ~9 % of the currently ~650 recorded species of the subcontinent (Patton et al. 2015). The number and identity of species listed may change owing to distinct reasons, in particular as result of both field and taxonomic work. As such, the list provided here is provisory and prone to change in the near future. Having said that, we expect that the general trends discussed here would remain for several years.
The species of our list are not evenly distributed among rodent families. Most belong to the family Cricetidae, a fact that it is not surprising since, in South America, this is the richest species rodent family (Patton et al. 2015) and by far the family were more new species are discovered (D’Elía et al. 2019a). Moreover, of the three cricetid subfamilies found in South America, none of the listed species belong to Neotominae nor Tylomyinae, but all to Sigmodontinae. The second family with more representatives is Ctenomyidae. No species from our list belongs to the hystricomorph families Erethizontidae, Dinomyidae, Dasyproctidae and Cuniculidae, nor the sciuriomorph Sciuridae and the supramyomorph Heteromyidae.
Amori et al. (2016) listed 30 South American rodent species only known from their type localities. Our list includes several restricted species described after Amori et al. (2016) closed their data compilation (i. e., 2005), but also several geographically restricted species, such as Akodon mystax and Oxymycterus caparoae, omitted by Amori et al. (2016). In addition, some species listed by Amori (2016) were not included in our list. This fact is consequence of changes prompted by recent taxonomic work (e. g., Akodon aliquantulus is now considered a synonymy of A. caenosus; Juscelinomys guaporensis is now considered a synonym for J. huancachae) and because known distributions have been redefined (e. g., Oecomys cleberi is now recognized as a widely distribute species in the southern portion of the Brazilian Cerrado; Patton et al. 2015).
Even when our criterion for inclusion species in the list is clear, some uncertainties persist regarding some species. One of these is Ctenomys brasiliensis, the type species of the genus Ctenomys. We include it in our list indicating it comes from the Uruguayan grasslands; however, the specific collection locality of the single specimen is unknown (Bidau 2015). Traditionally, C. brasiliensis whose collection locality data is consigned as “des parties intérieures du Brésil, de la Province de Las Minas” has been considered as collected in Minas Gerais, Brazil. However, not specimen of Ctenomys is known from that Brazilian state, while the holotype of C. brasiliensis falls in the morphospace of C. pearsoni, an Uruguayan species that inhabits a general area close to the Uruguayan city of Minas (Fernandes et al. 2012). In addition, at the time of collection, what is now Uruguay was part of the Brazilian Empire. As such, tentatively C. brasiliensis is considered as an Uruguayan form whose distinction of C. pearsoni should be further evaluated (Bidau 2015).
The taxonomic distinction of some of the species included in the list is doubtful and, as such, their presence in our list depends on the results of future taxonomic assessments. One of such case is the leaf eared mouse Phyllotis bonariensis, a form geographically restricted to the hilly system of Ventania in central-eastern Argentina (Steppan and Ramírez 2015). While some authors maintained this nominal form as a distinct species (being the reason why it is included in our list), others had suggested its conspecificity with P. xanthopygus, a species widely distributed in western South America, from Peru to Argentina and Chile (e. g., Teta et al. 2018). Another example is that of the vizcacha Lagostomus crassus, that may represent an extinct Peruvian population of L. maximus (Spotorno and Patton 2015). As an extreme case, it is possibly that the three supposedly endemic species of Brucepattersonius from Argentina (B. guarani, B. misionensis, and B. paradisus) could be considered as synonym of B. iheringi, a taxon currently distributed in forested areas of southern Brazil (Lanzone et al. 2018).
Several reasons could interplay to cause the rarity and/or the absence of recent records for some rodent species; some of them would be ultimately because of the lack of enough work and others are derived from biological attributes of the species (Amori et al. 2016; Meiri et al. 2017). For those recently described taxa, rarity could be an artifact of the lack of knowledge, as perhaps not enough time has elapsed for researchers to study them, including their distributional ranges (Meiri et al. 2017). If additional fieldwork is conducted, is likely that the known distribution of some species would be enlarged. For example, the fish-eating rat Neusticomys mussoi was only known from its type locality in western Venezuela since its description in 1991, being subsequently found at two additional localities in Venezuela and Colombia in 2008 and 2014, respectively (Rodríguez-Posadas 2014). A more eloquent example is that of the Kerr’s Atlantic forest rat, Phyllomys kerri, that being described in 1950, it was not rediscovered until 68 years later (Abreu-Junior et al. 2018). This could be certainly the case of other species in our list, since most of them were described in the last 50 years.
As mentioned above, even when field work is conducted, some biological attributes of the species may hamper the registry of new recording localities, including the fact that some species i) could be difficult to observe and collect due to their size, habits (e. g., climber, fossorial), or for being microhabitat specialists; ii) could be difficult to distinguish from other species and even when specimens are collected they are misidentified; iii) could have low populational densities; or iv) could be extinct (Amori et al. 2016; Meiri et al. 2017). Most species in our list are fossorial (e. g., Ctenomys) and some of them are climber (e. g., Phyllomys), which make them more difficult to catch trough traditional trapping procedures (Patterson 2002). Two species within our list, Juscelinomys candango and Lagostomus crassus, are considered extinct by the IUCN Red List; unfortunately, this number could increase in the next years. This could be the case of the water rat Nectomys saturatus, which is not observed since 1897 and that has lost most of its habitat owing to growing urbanization and desiccation of the meadows at its type locality (Chiquito and Percequillo 2019).
Most species in our list are found at or close to the Andes, including both forested and desert regions. Thus at least partially, the rarity of some of them could be related to the relative inaccessibility of medium to high altitude Andean areas. This could be the case of the mice of the genus Thomasomys, which in addition includes several species that easily confound among them (Pacheco 2015). Montane areas are usually complex geographical systems, in which speciation and microendemism are promoted by physical barriers and vertical succession of habitats (Maestri and Patterson 2016). This could be also the case of the Serra do Mar, in southeastern Brazil, which is included, together with the western Andean ranges, within the high richness areas for rodents in South America (Maestri and Patterson 2016).
At least four species in our list are island endemics; these are Cavia intermedia from Moleques do Sul Islands (Santa Catarina, Brazil; Cherem et al. 1999), Geoxus lafkenche from Guafo Island (Los Lagos, Chile; Teta and D’Elía 2017), Octodon pacificus from Mocha Island (Bio Bio, Chile; Hutterer 1994), and Phyllomys thomasi from Ilha de São Sebastião (Sao Paulo, Brazil; Emmons et al. 2002). Frequently, island endemics are more threatened than their continental counterparts owing to their usually smaller distributions, as well as facing habitat loss and introduction of alien species (Amori and Clout 2003). The four island endemics identified here fall in this trend; the tree of them that have been categorized by the IUCN are listed as CR (Cavia intermedia and Octodon pacificus) and EN (Phyllomys thomasi). We note that none of the endemic oryzomyine species from Galapagos Islands have distributional ranges restricted to their type localities, at least as is here defined; however, all of them occupies small geographical ranges, and are considered as VU (Aegialomys galapagoensis, Nesoryzomys fernandinae, N. narboroughi, N. swarthi) or even EX (N. darwini, N. indefessus).
As in previous contributions focused on geographically restricted taxa, most species in our list are considered as DD in the IUCN Red List (Figure 4), even when the use of this category is explicitly discouraged by the IUCN (IUCN 2017). Amori et al. (2016) suggested that this situation reflects a bias produced by the heterogeneity of assessors of the IUCN and the generalized assumption among researchers that extremely rare species are the consequence of suboptimal research efforts rather other causes. This situation is not exempt of consequences; species listed as DD usually do not gain much attention (i. e., conservation programs, founds) as those considered as CR, EN or VU (Amori et al. 2016). Almost one fourth of the species in our survey are included under one of the highest three categories of threat defined by the Red List (e. g., CR, EN, VU). Remarkably, there are more caviomorph than sigmodontine rodents on that list, perhaps because vizachas, chinchilla rats, and their relatives are more charismatic than mice and rats. Amori et al. (2016) draw attention to the points discussed here, suggesting the need to designate as CR all species geographically restricted to their type localities, at least when there are no recent records (i. e., in the last 25 years).
The identification of those species with the smallest ranges is important both from an ecological and conservational perspective (Meiri et al. 2017). On the one hand, most of the geographically restricted species may be functionally analogous to “singletons” in ecological communities, being mostly unknown in their basic aspects of their natural history (e. g., diet, movements, reproduction). True narrow endemic species are also pivotal to understood biogeographical processes (Meiri et al. 2017). On the other hand, considering these species is crucial to develop adequate conservation strategies and to decide how to allocate finite resources (Amori et al. 2016). As such, the species list provided here needs to be depurated trough additional field and collection based work. Even when some species could be removed from this list, it is also possible that others could be included. We close this contribution by stating that we expect that our list and the considerations expressed will help draw attention to those poorly known South American rodent species, triggering the desire to conduct research on them. Also, we expect that authorities and agencies granting funds and authorizations to conduct field work understand the importance of this activity towards a better knowledge on these species and ultimately towards their conservation (see Thomson et al. 2018 and D’Elía et al. 2019b).











 nueva página del texto (beta)
nueva página del texto (beta)


