Introduction
Two genera of shrews inhabit northern Central America: Sorex and Cryptotis. The small-eared short tailed shrews of the genus Cryptotis occur from southern Canada, through the United States, México and Central America, to northwestern South America (Choate 1970). There are at least 42 known species in four species-groups based on morphological and molecular characters (He et al. 2015; Hutterer 2005; Guevara et al. 2014), but their taxonomy still remains subject to many changes. Three groups and seven species are known for Guatemala: C. goodwini, C. lacertosus, C. mam, C. oreoryctes, C. tropicalis, C. mayensis, C. merriami (Hutterer 2005; Carraway 2007; Woodman 2010). The C. nigrescens group includes seven tropical and mountain species from México to the Andes, but only two are known for Guatemala: C. mayensis and C. merriami (Woodman and Timm 1993). Recently Guevara et al. (2014) described C. lacandonensis, a lowland short-tailed shrew of the C. nigrescens group, from the Lacandona rain forest at Yaxchilán archaelogical site, in Chiapas, México (Figure 1). C. lacandonensis is the sister species of C. mayensis, based on molecular information (Guevara et al. 2014, He et al. 2015), and they are the only two species known to occur in the lowlands of the Yucatan Peninsula in México, the Petén in Guatemala, and Belize. C. mayensis is widely distributed in the Yucatán Peninsula, while C. lacandonensis is currently known only from the type locality, on the eastern and Mexican shore of the Usumacinta River. This is the longest and largest river of northern Central America, and the portion that serves as the border line between México and Guatemala has a very rainy climate, which differs from the common dryer areas of the Yucatán Peninsula.
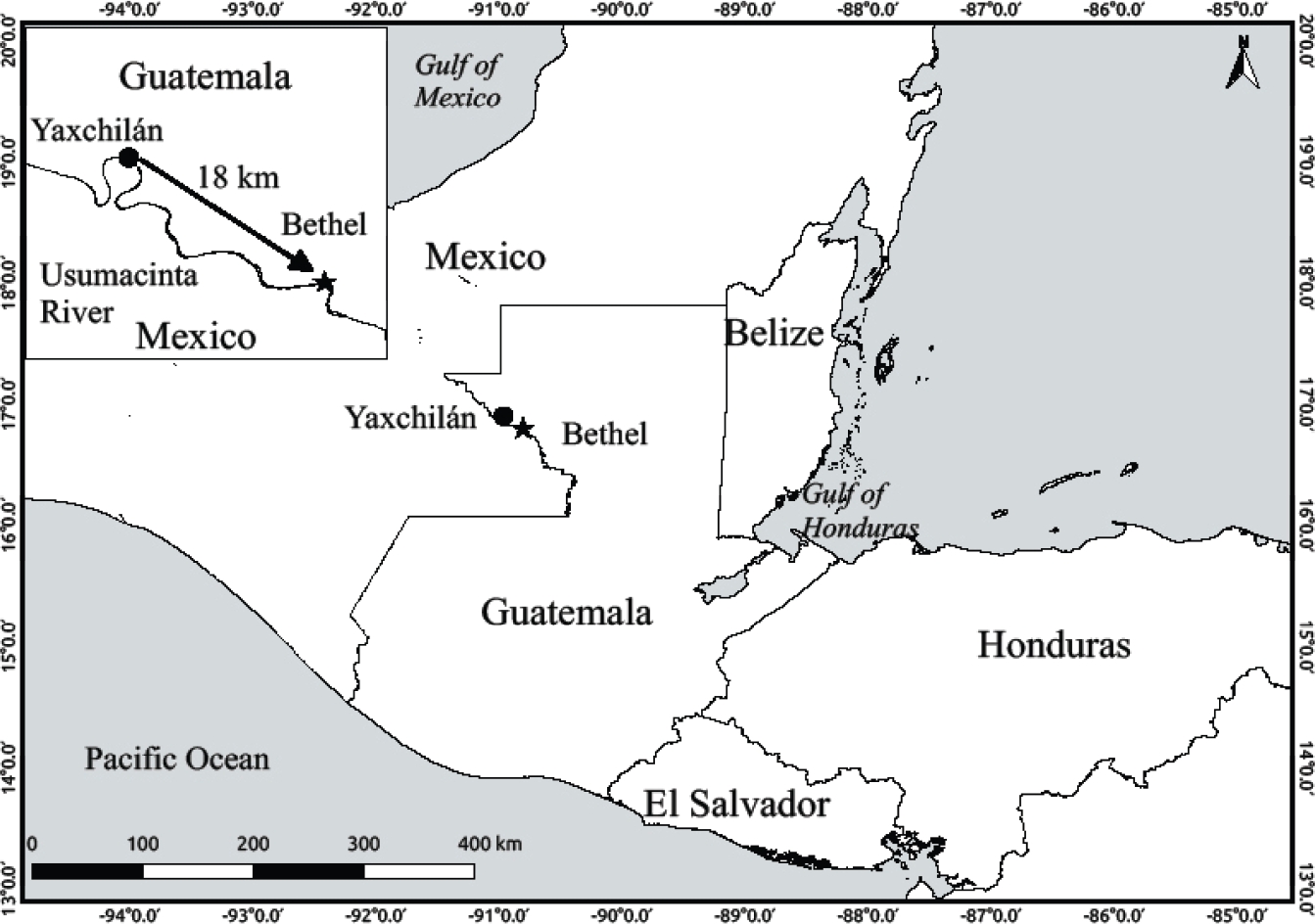
Figure 1 Map of Guatemala and adjacent areas showing the locality at the Community of Bethel, Petén, Guatemala, where “Bethel specimen” was collected, and the type locality of Cryptotis lacandonensis at Yaxchilán Archeological site in nearby Chiapas, México (taken from Guevara et al. 2014).
The revision of an unidentified specimen of shrew deposited at the mammal collection of San Carlos University in Guatemala City put us the task of identifying it. The specimen comes from the area of the Usumacinta River on the side of Guatemala, at the community of Bethel, Petén. With that purpose we conducted comparisons with a series of shrews from the lowland and highlands of nearby areas, both morphological and morphometrically, with the main target of resolving this identification problem.
Material and methods
On November 19, 1995 one of us (MRJ) collected one specimen of shrew while conducting a small mammal inventory research around the community of Bethel, 74 km W La Libertad, Department of Petén, Guatemala, 130 m, ca. 16.8267°N, -90.8122°W (Figure 1). The specimen is an adult female captured with a Sherman trap (field number MRJ 103), preserved in fluid-alcohol, and deposited at the mammal collection at Universidad de San Carlos de Guatemala (USAC 6066, Figure 2), without any identification to the species level. We will refer to this specimen later on as the “Bethel specimen”.
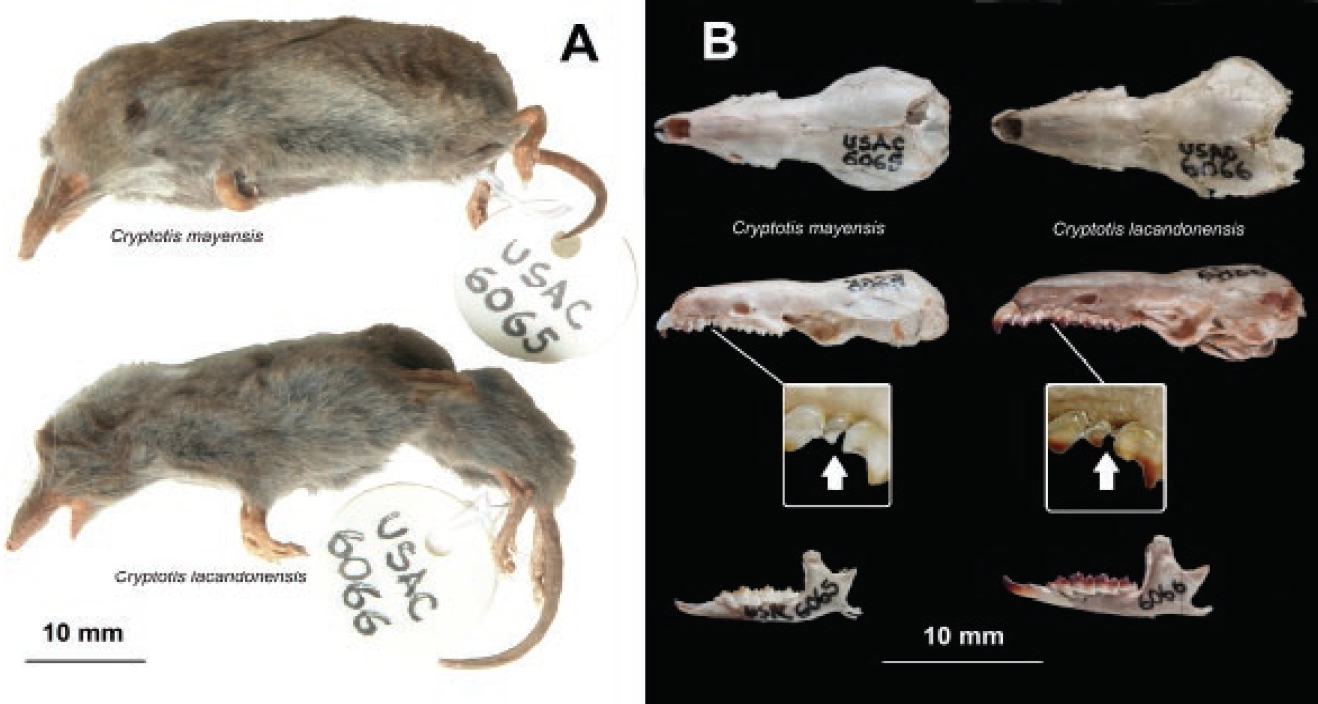
Figure 2 A: fluid preserved specimens of Cryptotis lacandonensis USAC6066 (down) and C. mayensis USAC6065 (up). B: skulls of Cryptotis lacandonensis (right) and C. mayensis (left) in dorsal, lateral, and lateral mandibles (down); inserted are magnifications of labial views of the upper fourth unicuspid teeth (U4).
The area of Bethel is located at the southernmost limit of the Sierra del Lacandón National Park, which is also part of the Mayan Biosphere Reserve. The area was at that time covered mainly by humid tropical forest and seasonally flooded areas, but crops and cattle now dominate it. Nonetheless, pristine forest is still found in nearby wild protected areas both in México and in Guatemala (Méndez 1997). The original forest is classified as “high evergreen jungle” based on Miranda and Hernandez-X classification (Challenger and Soberón 2008), with different habitats locally known as “planicies” (tall forests on non-inundated plains), “bajos” (swamps with short forest), and “serranías” (tall forest on hills). Common species of trees in the area include: Brosimum alicastrum Sw. 1788, Brosimun costaricanum Liebm. 1851, Pouteria reticulata (Engl.) Eyma 1936, Alseis yucatanensis Standl. 1930, Coccoloba barbadensis Jacq. 1760, Blomia prisca (Standl.) Lundell 1961, Terminalia amazonia (J. F. Gmel.) Exell 1935, Coccoloba barbadensis Jacq. 1760, Simaruba amara Aubl. 1775, Calophyllum brasiliense Cambess. (1828) among others (Méndez 1997; Soto 2003).
With the target of correctly identify this specimen, we recently extracted the skull of this fluid preserved specimen and then cleaned it with the help of a dermestid beetles’ culture, and then with a soft solution of ammoniac. For comparative reasons, we also examined 20 additional skulls within the C. nigrescens group (C. merriami and C. mayensis), C. goldmani group (C. mam and C. goodwini), and C. parva group (C. tropicalis). These specimens are deposited in two collections: Universidad de San Carlos de Guatemala (USAC) and Museo de Historia Natural, Ciudad Universitaria, Universidad Nacional Autónoma de Honduras (UNAH-CU); a complete list of specimens examined and their localities is given in the forward section. We were not able to check directly the holotype and paratype of C. lacandonensis, as it is desired in this comparisons, but since it was one of the probable names for the Bethel specimen, due to the proximity to the type locality, we included in the morphometric analysis the measurements of the holotype and paratype reported in Guevara et al. (2014).
We obtained 13 cranial measurements of all skulls following Woodman and Timm 1993: condylobasal length, not including the upper incisors (CBL); cranial breadth (CB); breadth of zygomatic plate (ZP); interorbital breadth (IO); breadth of palate across first unicuspids (U1B); breadth of palate across third unicuspids (U3B); breadth of palate across second molars (M2B); palatal length (PL); upper tooth row length, Ul to M3 (TR); molariform tooth row length, P4 to M3 (MTR); height of coronoid process (HCP); height of coronoid valley (HCV); lower tooth row length, length p3 to m3 (TRD). All measurements were recorded in millimeters to the nearest 0.1 mm using an electronic caliper TRUPER®. For the majority of specimens examined, and when available, we took note of standard external measurements recorded by the collectors in the field, with the majority written in their museum tags or recorded in collections data bases: total length (TL), length of tail (LT), and hind foot (HF), in millimeters. Sex of specimens was that recorded on tags or by field notes, or when possible, determined in the laboratory following Searle (1985).
We conducted a Principal Component Analysis (PCA) and a Linear Discriminant Analysis (LDA) with craniodental measurements, to see if the Bethel specimen groups with any of the known shrew specimens of the area. We used transformed Log10 measurements to reduce the excessive weight of some of the variables (Sokal and Rohlf, 2012). External measurements were not included in the morphometric analysis. Because of the limited number of specimens examined and to avoid saturation of the analysis, we explored pair correlations of variables (results not shown) and avoided to use the most redundancy ones. We decided to use only eight selected variables for PCA and LDA analysis. We also confirmed normality of all variables. Both PCA and LDA included 23 individuals in total (Bethel specimen plus 20 specimens examined and two type specimens reported in Guevara et al. (2014).
All calculations were performed in R version 3.4.1 (R Development Core Team 2017). PCA was finally performed with 23 individuals and eight selected variables (CBL, CB, ZP, M2B, MTR, TR, and TRD), using the package FactoMineR (Lê et al. 2008). We then evaluated the conformation of the groups (species) a priori considered in this study through a Linear Discriminant Analysis (LDA) with the same eight variables and to be able to evaluate the Bethel specimen as part of C. lacandonensis; this was done with the package MASS (Venables and Ripley 2002). We then plotted the first two components of PCA and LDA with ggplot2 (Wickham 2016).
Specimens examined:
Bethel specimen (Cryptotis cf. lacandonensis): GUATEMALA: Comunidad Bethel. Las Cruces, Petén, 122 m, 16.8219°N, -90.8036°W (1F, USAC6066).
C. mayensis: GUATEMALA: Sylvanus Morley Museum, Tikal National Park, Flores, Petén, 246 m, 17.2257° N, -89.6126° W (1F, USAC6065).
C. merriami. HONDURAS: Parque Nacional La Tigra, Francisco Morazán 14.8506°N, -87.5201°W (MHN-CU 20160020, MHN-CU 20150015). GUATEMALA: Aldea El Duraznal, Trifinio National Park, 1.6 km S, 7 km E Concepción Las Minas, Chiquimula, 1,655 m, 14.5063° N, -89.3804° W (1M, USAC5864); Finca Biotopin, 2.3 km S, 1.6 km E Purulhá, Baja Verapaz, 1,705 m, 15.2168333° N, 90.2181389° W (1 sex undetermined, USAC 5866).
C. goodwini: GUATEMALA: Finca Barillas, 11.5 Km N, 1.1 Km W Colomba Costa Cuca, Quetzaltenango, 1,680 m, 14.8130° N, -91.7405° W (2F, USAC5661, USAC5680); Volcán Acatenango, ca. 1.6 km S, 6.4 km E Acatenango, Chimaltenango, 3,000 m, 14.5211° N, -90.8797° W (1F, USAC1037); Parque Ecológico Cayalá, zona 16, Ciudad de Guatemala, Guatemala, 1,451 m, 14.6199° N, -90.4893° W (2M, USAC1021, USAC0059); Finca El Pilar, Magdalena Milpas Altas, 11 km SE Antigua Guatemala, Sacatepéquez, 2,640 m, 14.5180° N, -90.6919 (1M, USAC5870); Cumbre de Montecristo, Esquipulas, Chiquimula, 2,400 m 14.4192° N , -89.3558, (3F, USAC3325, USAC3319, USAC3334)); Las Minas, Los Planes, Chiquimula, (1, sex undetermined, USAC2384).
C. mam: GUATEMALA: San Francisco, El Retiro, Cuilco, Huehuetenango, 2,975m. 15.4854° N, -91.9327° W (1F USAC5748).
C. tropicalis: GUATEMALA: Cerro el Gigante, 5.3 km N, 13.4 Km W Chiquimula 1745 m, 14.8458° N, -89.6667° W (2M USAC5426, USAC5443); Parque Ecológico Cayalá, zona 16, Ciudad de Guatemala, Guatemala, 1451 m, 14.6199°N, 90.4893°W (2 sex undetermined, USAC1020, USAC 1019).
Results
A morphological comparison of skulls showed that the Bethel specimen (USAC 6066) is similar to specimens of C. merriami from the mountains southward in Guatemala, but they are differentiated by the general bigger size of C. merriami, which also has a bigger maxillary U4 tooth and much wider gap between U3 and P4, giving more room to U4. In comparison with C. mayensis (USAC 6065), the Bethel specimen is bigger, and also can be differentiated by the presence of a medium size U3-P4 gap (but not as wide as in C. merriami), which is wide enough to let medium tooth U4 partially visible in labial view of the skull (see Figure 2B, magnification inserted, see arrow); in C. mayensis, U3 and P4 appear almost in touch, with a small and intermediate U4 hidden in lateral view (Figure 2B). Externally, both C. mayensis and Bethel specimen show a general dark grayish pattern, but C. mayensis from Tikal is slightly more brownish in the dorsal pelage (Fig. 2A).
External and cranial measurements of the Bethel female specimen (USAC 6066) are as follows [incomplete or estimated measurements in brackets]: TL 92, LT 27, HF 12; CBL [18.40], CB 8.80, ZP 2.44, IO 4.48, U1B 2.16, U3B 2.68, M2B 5.19, PL 8.35, TR 7.22, MTR 5.26, HCP 5.39, HCV 3.03, TRD 5.61. Measurements of this and all other specimens included in the analysis are shown in Table 1.
Table 1 Ranges, means and standard deviation (in parenthesis) recorded for different species of Cryptotis from Guatemala, Honduras and Chiapas, including Bethel specimen and reported measurements of type specimens of C. lacandonensis taken from Guevara et al. (2014). Abbreviations of variables follow those described in text.
| C. goldmani group | C. parva group | C. nigrescens group | |||||
|---|---|---|---|---|---|---|---|
| Variables | C. goodwini (n = 10, except external measurements) |
C. mam (n = 1) |
C. tropicalis (n = 4 except external measurements) |
C. merriami (n = 4, except external measurements) |
C. mayensis (n = 1) |
Bethel specimen (n = 1) |
C. lacandonensis (from Guevara et al. 2014, n = 2) |
| HB | 70.00-96.00 84.28 (9.21) (n = 7) |
82 | 54, 59 (n = 2) |
78, 83 (n = 2) |
57 (from fluid specimen) |
65 (from fluid specimen) |
78, 81 |
| LT | 26.00-31.00 29.00 (2.00) (n = 7) |
29 | 22, 22 (n = 2) |
24, 32 (n = 2) |
23 (from fluid specimen) |
27 (from fluid specimen) |
33, 35 |
| HF | 13.00-15.00 14.14 (0.89) (n = 7) |
15 | 11, 11 (n = 2) |
11, 13 (n = 2) |
10 (from fluid specimen) |
12 (from fluid specimen) |
--- |
| CBL | 19.98-21.52 20.79 (0.59) |
21.45 | 16.49-17.51 16.94 (0.42) |
18.5-19.73 18.94 (0.56) |
17.58 | 18.4 | 20.1, 20.5 |
| CB | 10.42-11.13 10.75 (0.22) |
9.87 | 7.64-8.85 8.25 (0.60) |
9.20-9.71 9.43 (o.26) |
8.17 | 8.8 | 9.6, 9.7 |
| IO | 5.3-5.84 5.47 0.17 |
5.5 | 3.94-4.33 4.12 (0.17) |
4.55-4.86 4.64 (0.14) |
3.9 | 4.48 | 4.7, 4.8 |
| ZP | 1.87-2.39 2.12 (0.16) |
2.53 | 1.44-1.91 1.69 (0.22) |
2.07-2.31 2.21 (0.11) |
2.31 | 2.44 | 2.7, 2.4 |
| PL | 8.49-9.24 8.83 (0.30) |
8.85 | 6.47-7.28 6.82 (0.39) |
7.75-8.50 8.09 (0.39) |
7.67 | 8.35 | 9.1, 9.1 |
| M2B | 5.81-6.24 6.08 (0.12) |
6.08 | 5-5.37 5.18 (0.17) |
5.41-4.45 5.43 (0.01) |
4.89 | 5.19 | 5.5, 5.4 |
| U1B | 2.55-3.18 2.69 (0.18) |
2,61 | 2.01-2.40 2.21 (0.19) |
2.19-2.41 2.44 (0.23) |
2.15 | 2.16 | 2.6,2.6 |
| U3B | 3.07-3.41 3.23 (0.11) |
3.05 | 2.53-2.78 2.7 (0.11) |
2.70-3.17 2.95 (0.19) |
2.42 | 2.68 | 3.1, 3.0 |
| MTR | 5.23-5.78 5.5 (0.17) |
5.46 | 4.18-4.56 4.34 (0.18) |
4.99-5.49 5.18 (0.22) |
4.88 | 5.26 | 5.3, 5.2 |
| TR | 7.44-7.98 7.69 (0.19) |
7.91 | 6-6.28 6.1 (0.13) |
7.12-7.76 7.37 (0.29) |
6.51 | 7.22 | 7.7, 7.7 |
| TRD | 5.94-6.5 6.26 (0.20) |
6.47 | 4.75-5.05 4.91 (0.14) |
5.35-5.80 5.57 (0.18) |
5.42 | 5.61 | 5.6, 5.8 |
| HCP | 4.64-5.19 4.83 0.16 |
4.78 | 4.01-4.27 4.14 (0.11) |
4.51-4.97 4.73 (0.18) |
4.41 | 5.39 | 5.0, 5.2 |
| HCV | 2.87-3.4 3.07 (0.17) |
3.12 | 2.37-2.63 2.48 (0.13) |
2.65-2.99 2.80 (0.16) |
2.5 | 3.03 | 2.8, 2.7 |
External measurements of the Bethel specimen are smaller from those reported for the types of C. lacandonensis in Guevara et al. (2014), but much bigger in comparison with the specimen of C. mayensis (USAC 6065) measured with the same method (see Figure 2A). Skull measurements are similar (but slightly smaller) from those reported for C. lacandonensis; and in comparison with C. mayensis they are generally bigger.
Principal component analysis (Figure 3A) showed that the first two axes explains 90 % of the variation (PC1 75.23 %, PC2 14.77 %). The measures CBL, TRD and IO were the ones with the highest scores and contributions to PC1, meanwhile ZP and HCP were the ones with the highest contributions to PC2 (see factor loadings shown in Table 2). The PCA showed that bigger size and highland species of the C. goldmani group (C. goodwini and C. mam) were located at the bottom-right side of the graphic, and smaller lowland species of the C. nigrescens group (C. merriami, C. mayensis, and C. lacandonensis) and parva group (C. tropicalis) were placed on the left side of the graphic (Figure 3A). Bethel specimen (showed only as “Bethel”) appeared close but not well grouped with C. lacandonensis.
Table 2 Factor loadings from PCA and LDA for axes 1 and 2
| Variables | PCA | LDA | ||
|---|---|---|---|---|
| PC1 | PC2 | LD1 | LD2 | |
| CBL | 0.3931 | -0.0424 | -0.3657 | 0.2382 |
| TRD | 0.3873 | -0.0168 | 0.5275 | 0.0338 |
| IO | 0.3836 | -0.2729 | 0.2515 | 0.4516 |
| M2B | 0.3705 | -0.3442 | 0.1851 | -0.6381 |
| HCV | 0.3702 | 0.0552 | 0.3595 | -0.2133 |
| U3B | 0.3513 | -0.2308 | 0.0947 | -0.0838 |
| HCP | 0.3140 | 0.4877 | -0.5729 | 0.4730 |
| ZP | 0.2279 | 0.7147 | -0.1555 | 0.2342 |
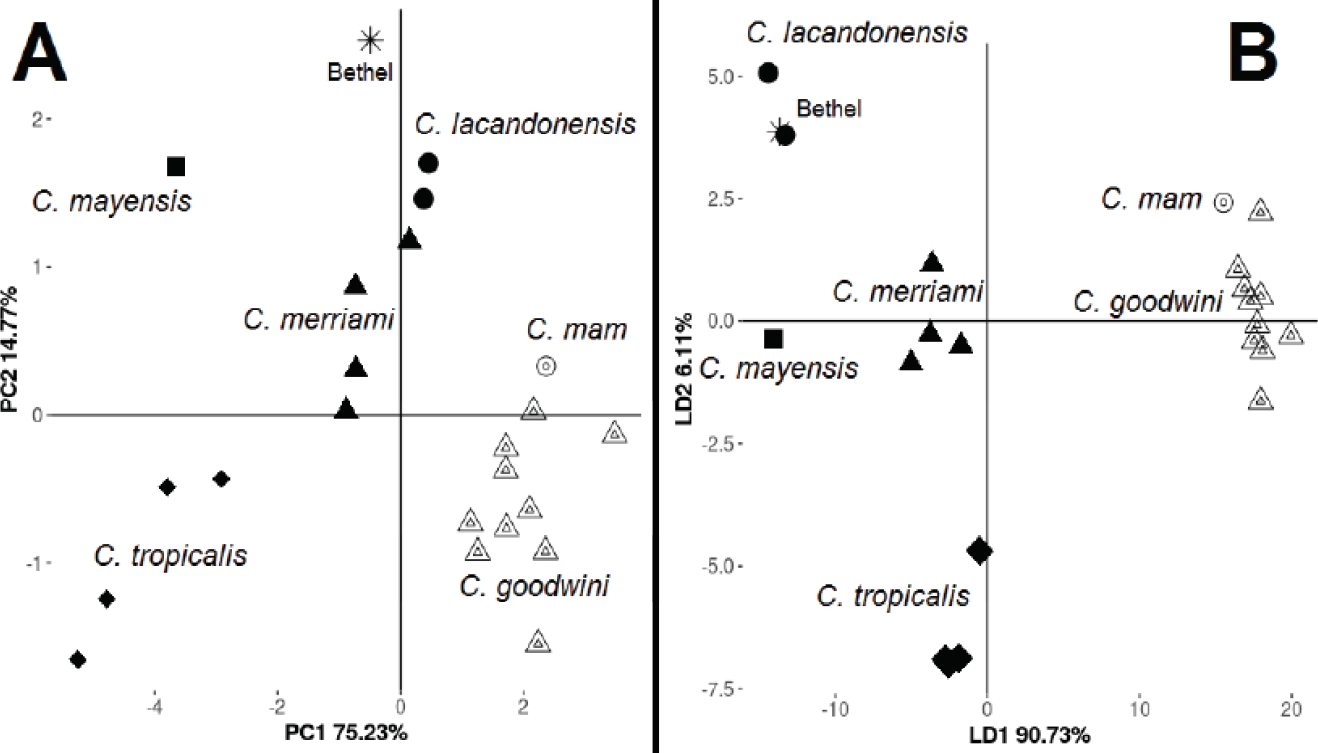
Figure 3 Scatter plot of principal component analysis (A) and linear discriminant analysis (B) for different species of Cryptotis from Chiapas, Guatemala and Honduras. Bethel specimen is marked with an asterisk.
LDA results (Figure 3B) are in general in accordance with PCA, with highland species (C. goodwini and C. mam) at the right side of the graph, and lowland species well discriminated at the bottom (C. tropicalis), center left (C. merriami and C. mayensis), and top-left (C. lacandonensis and Bethel specimen). In general, LDA was better to discriminate species and groups than PCA, placing the Bethel specimen decidedly closer to C. lacandonensis than to C. mayensis. In LDA, the first function explains 90.73 % of the variance, with higher weights for HCP and TRD (see Table 2 for factor loadings), while the second function explains the 6.11 % of the variance with higher weights for M2B and HCP. LDA showed the conformation of well-defined groups, represented by the a priori species designations, and the classification function was 100 % in accordance with these designations.
Discussion and Conclusion
Habitat of the community of Bethel is practically the same to that of the type locality at Yaxchilán, Chiapas, México. The majority of species of the genus Cryptotis inhabit the mountains of southern Nuclear Central America, with only two species occurring in the lowlands of the Yucatán Península, C. lacandonensis and C. mayensis. Nonetheless, C. mayensis appears to be restricted to dryer and northernmost areas of the peninsula (Cuarón et al. 2016), with no overlapping distribution with C. lacandonensis. The forest at Tikal, for example, is classified as “high sub-evergreen jungle” (Challenger and Soberón 2008), with more seasonality during the long dry seasons, and with very little surface water run-off. The vegetation at Tikal is similar to Bethel, but is more dominated by Brosimum, Swietenia, Cedrella and other dry forest species (Schulze and Whitacre 1999; Jolón 1996). So the distribution of C. lacandonensis was expected to extend around the neighbor humid lowlands areas of the type locality at the Usumacinta River (Guevara et al. 2014).
Because of the general morphology, teeth pattern, coloration, and results of the PCA and LDA analysis, we conclude that Bethel specimen can be determined as Cryptotis lacandonensis. Nonetheless, not all information fit perfectly with the description of the species, because the external measurements are smaller from those of the type locality, although we are aware of the potential differences that may be due to many factors. These factors include the way the measurements were taken, for example if taken in the field or from preserved specimens, and also differences due to sex, age or regional variations that we simply do not know about.
The Bethel specimen represents a small range distributional extension for the species, but the first record for Guatemala. C. lacandonensis remains as an endemic species to the lowlands of the Usumacinta River basin, with potential as species flag for conservation porpoises on both sides of the Mexican-Guatemalan border. Life history and ecology is mostly unknown, and research on these aspects is still needed. Due to its known restricted distributional extension and the rapidly fragmentation of the original forest (mainly in Guatemala), this small mammal is a good candidate to be considered in threat, but its classification as “data deficient” is good for now.
The evolutionary history of the C. nigrescens group in Nuclear Central America still needs to be untangled. We do not know how and when the divergence of C. merriami, C. mayensis and C. lacandonensis occurred. The first species is relatively common at mid elevations at the southern side of Nuclear Central America, and shows a complex variation that in the future could be clue to resolve many of the present doubts.
At present, the area of the Usumacinta River, especially on the side of Guatemala, is suffering a drastic forest loss and increase of human population. We do not know if this species will be able to adapt and survive to this new scenario. Fortunately, there are important efforts of conservation of nature being held on both sides of the border in México and Guatemala and we hope that this rare species gets more attention.











 nueva página del texto (beta)
nueva página del texto (beta)


