Introduction
One of the main attributes of an ecological niche is the feeding resource associated with the species (Patterson et al. 2003). For bats, this component is one of the principal mechanisms associated with resource partitioning within assemblages (Giannini and Kalko 2004; Sánchez and Giannini 2018). Importantly, bats are known dispersers of seeds, and owing partly to their degree of diet specialization, maintaining intact bat assemblages has important implications for forest restoration and recovery following disturbance (Reid et al. 2015). For instance, within Neotropical frugivorous bats, species of the genera Artibeus, Carollia and Sturnira seem to specialize on different fruit species (Fleming 1986; Giannini and Kalko 2004; Saldaña-Vázquez 2014; Saldaña-Vázquez et al. 2015). Thus, 67 % of the Artibeus diet is composed of Ficus and Cecropia fruit, 60 % of the Carollia diet is made up of Piper fruit, and more than 50 % of the Sturnira diet is composed of Solanum fruit (Saldaña-Vázquez et al. 2013). Nevertheless, this pattern has been described mainly in tropical moist forests (Fleming 1986; Giannini and Kalko 2004; Lobova et al. 2009), and current studies conducted in other Neotropical ecosystems have shown different dietary characteristics (Ríos-Blanco and Pérez-Torres 2015).
The Orinoco region of Colombia, also known as the Eastern Llanos, holds 114 bat species, which represent 55 % of the species in the country (Pardo and Rangel 2014). Oil palm has become a main agricultural activity in this region, with palm plantations covering 206,559 ha (40.3 % of palm production of the country; Fedepalma 2017). Moreover, since current government projections suggest an expansion of oil palm cultivation in Colombia to approximately 1,000,000 ha in 2020 (Ministerio de Agricultura y Desarrollo Rural 2006), oil palm coverage probably will increase in the future. However, studies related to the impact of oil palm agriculture on biodiversity are scarce for the country (Pardo et al. 2015).
Considering the future restoration planning required by the government after oil palm production (Ministerio de Ambiente y Desarrollo Sostenible 2015), dietary analysis is an important step toward understanding the future seed rain in these environments, which will influence the local vegetation of early successional ecosystems (Mikich 2002; López and Vaughan 2007). Our objective was to characterize the diets of the dominant frugivorous bat species, Artibeus lituratus, A. planirostris, Carollia spp., and Sturnira lilium, in an oil palm landscape from the Colombian Llanos.
Materials and methods
Study area. The study was carried out in Hacienda La Cabaña plantation, an oil palm agricultural area of 2,200 ha from Cumaral municipality, in Meta Department, situated in the Llanos Orientales Region of Colombia (centroid of study area: 4° 18’ 18.4” N, -73° 21’ 26.5” W), between 310 and 368 meters elevation. Included in this landscape mosaic are remnants of primary and secondary forest, cattle pastures, and gallery forest along waterways.
Bat sampling. During January and February 2016 (at the end of the dry season), three palm crops of different height categories (5, 15, and 20 m) and one secondary forest in the palm matrix were selected for bat sampling. Due to the heterogeneity between the heights of palms and forest, a stratified sampling was carried out where the strata corresponded to the coverages: palm (5, 15 and 20 meters of height) and the forest. In each coverage seven mist nets (6 x 3 meters and 9 x 3 meters) were placed separated by at least 5 meters from each other using as a reference the furrows and palms arrangements in the crop, for three consecutive nights. We opened mist nets between 18:00 and 6:00 hr to capture bats. We individually marked each bat punch-marking numbers into their wing membranes with tattoo pliers for small domestic animals, to avoid repeat fecal samples from individuals. The total sampling effort was of 1,620m2 mist-net hrs.
We evaluated Carollia perspicillata and C. brevicauda together as Carollia spp., due to the difficulty of taxonomic identification of the species. This does not affect data analysis due to the known phylogeny in dietary specialization in frugivorous bats (Giannini and Kalko 2004; Saldaña-Vázquez 2014; Sánchez and Giannini 2018).
We obtained fecal samples two ways. First, after capture, frugivorous bats were placed in a cloth bag for approximately 30 minutes to collect fecal samples. Second, we placed a plastic sheet below mist nets to collect the feces that fell to the ground at the time of capture. Plastic sheets were cleaned every 30 minutes, each time mist nets were checked. Fecal samples were stored in Eppendorf tubes with 70 % alcohol, labeled with the individual’s capture code, and processed according to Mello et al. (2004). The seeds were identified from comparisons with the seed collection of the Museo Javeriano de Historia Natural from Pontificia Universidad Javeriana (Bogotá, Colombia; MPUJ-MAMM-S), and seed taxonomic keys (e. g., Lobova et al. 2009; Linares and Moreno-Mosquera 2010).
Data analysis. The representativeness of seed species richness was calculated using Chao 2, which is a non-parametric estimator that provides the least biased estimates for small numbers of samples (Colwell and Coddington 1994), as in this study. Chao 2 also avoids problems related to detection probabilities and abundance estimation using incidence data (Pardo et al. 2018). For this, accumulation curves of observed species were constructed, and the count of new species was modeled with respect to the unit of sampling, the value of the estimated richness is the asymptote of the curve. The unit of sampling consisted of the fecal samples. To eliminate the effect of the order in which each fecal sample was added, we randomized sample order (n = 100) using EstimateS 9.1 (Colwell 2013). The representativeness percentage of seed species richness was calculated based on the richness estimated of Chao 2 estimator.
Regarding dietary analysis, we calculated the proportion of the consumption of seed species by the frugivorous bats based on the number of presences of each seed species in the total amount of fecal samples of each bat species. To determinate if the frequency of seeds consumed differed among the four bat taxa, we applied Chi-square tests (χ2), using a level of significance of 0.05. Levin’s index was used to describe the diet breadth of all the species (Levins 1968). We used Hulbert’s (1978) correction, to scale the diet breadth from 0 to 1 (B a), facilitating comparisons among species. In this scale, the value 0 represents that all individuals of a species consume the same food species (more specialist) and the value 1 indicates that all individuals consume the totality of food items that are available, more generalist (Hulbert 1978). Finally, we used the Morisita-Horn index to test diet overlap among frugivorous species (Horn 1966).
Results
Frugivorous bat assemblage. We captured 393 individuals from 18 species and three families. Carollia spp. was the most abundant taxon, followed by A. planirostris, A. lituratus and S. lilium (Table 1), from the four taxa 149 fecal samples were obtained. These frugivorous bat species constituted 87.5 % of the total individuals captured.
Table 1 Bat abundance, seed species richness and Levin’s index for frugivorous bat taxa found in Hacienda La Cabaña, Meta, Colombia. Proportion of total captures is given for the four dominant taxa.
| Species | Bat abundance | Seed species | Levin’s index |
|---|---|---|---|
| Artibeus lituratus | 14 (3.56 %) | 4 | 1.80 |
| Artibeus planirostris | 86 (21.88 %) | 9 | 2.63 |
| Carollia spp. | 233 (59.28 %) | 14 | 5.59 |
| Sturnira lilium | 11 (2.79 %) | 1 | 1 |
| Remaining bat species | 14 species (12.49 %) | 4 |
Diet of frugivorous bats. A total of 13 plant species along with two undetermined morphospecies were found in the fecal samples (Figure 1; Table 2). According to the Chao 2, we obtained a completeness over the 85 % of the seed species in our study (Figure 2) indicating an adequate sample with 93 % of representativeness of the majority of seed species (Sobs = 16; Chao2 mean = 17.19; Chao 2 SD = ±1.83). The most important plant species in bat diets were Cecropia peltata, Vismia macrophylla and Solanum aff. jamaicence (Table 2). The composition and amount of seed consumption was quite different among bat species (χ2 = 89.96, d. f. = 48, P < 0.05; Figure 3). Carollia spp. had the broadest diet (B a = 0.353), followed by A. planirostris (B a = 0.125), A. lituratus (B a = 0.06) and S. lilum (B a = 0.007). The highest diet overlap was between A. planirostris and A. lituratus, followed by the Artibeus and Carollia species (Table 3).

Figure 1 Photographs of seeds consumed by frugivorous bat species in Hacienda La Cabaña (Meta, Colombia). Scale bars = 1 mm.
Table 2 Percentage of plant species occurrence for four frugivorous bat species in Hacienda La Cabaña, Meta, Colombia.
| Family | Plant species | Bat species | |||
|---|---|---|---|---|---|
| A. lituratus | A. planirostris | Carollia spp. | S. lillium | ||
| Urticaceae | Cecropia peltata | 72.73 | 60.53 | 18.10 | 0 |
| Cecropia sp. | 0 | 2.63 | 0.86 | 0 | |
| Moraceae | Ficus insipida | 9.09 | 13.16 | 1.72 | 0 |
| Ficus sp. | 0 | 10.53 | 2.59 | 0 | |
| Piperaceae | Piper unillanum | 9.09 | 2.63 | 11.21 | 0 |
| Piper sp. 1 | 0 | 2.63 | 6.90 | 0 | |
| Piper peltatum | 0 | 2.63 | 4.31 | 0 | |
| Piper sp. 2 | 0 | 0 | 0.86 | 0 | |
| Clusiaceae | Vismia macrophylla | 0 | 2.63 | 27.59 | 0 |
| Solanaceae | Solanum aff. jamaicense | 9.09 | 2.63 | 21.55 | 0 |
| S. schlechtendalianum | 0 | 0 | 0.86 | 0 | |
| Solanum sp. | 0 | 0 | 0 | 100 | |
| Fabaceae | Fabaceae sp. 1 | 0 | 0 | 1.73 | 0 |
| Morphospecies 1 | 0 | 0 | 0.86 | 0 | |
| Morphospecies 2 | 0 | 0 | 0.86 | 0 | |

Figure 2 Accumulation curve of seed species consumed by frugivorous bat species at oil palm dominated landscape in Hacienda La Cabaña (Meta, Colombia). Grey solid line indicates Chao 2 model; black solid line represents the number of observed seed species (S = 16).
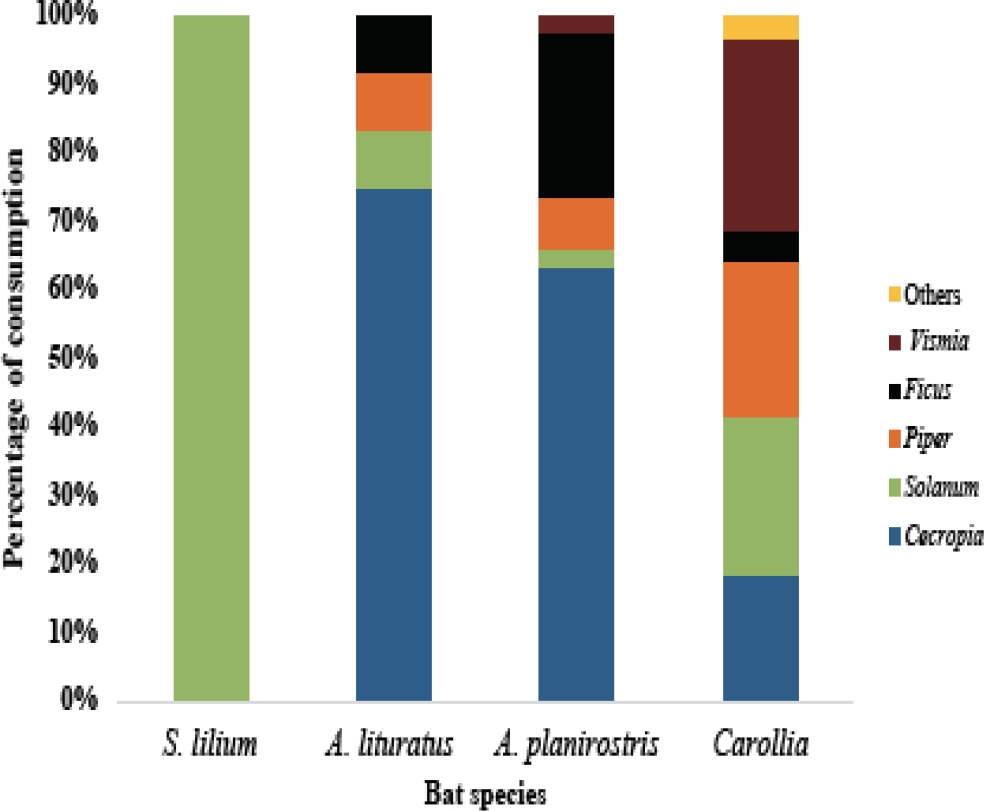
Figure 3 Proportion of consumption of each genus of plant by the different frugivorous bat species in Hacienda La Cabaña (Meta, Colombia).
Discussion
Diet variation among frugivorous bats. Differences in diet observed in this study, combined with low diet overlap (Table 2, Table 3, Figure 3), suggest niche segregation as a strategy to avoid competition among bat genera in our study area (López and Vaughan 2007; Saldaña-Vázquez 2014; Sánchez and Giannini 2018). We found that the main component of S. lilium diet was Solanum, a finding supported by prior studies (Fleming et al. 1986; Giannini and Kalko 2004; Saldaña-Vázquez 2014). In contrast, we observed a high incidence of Cecropia in the diet of A. planirostris and A. lituratus, bats previously reported to feed heavily on Ficus (Mikich 2002; Ríos-Blanco and Pérez-Torres 2015). This is not unexpected, as phyllostomid bats shift diet between plant genera depending on their availability (Fleming et al. 1986; Giannini and Kalko 2004; Gonçalves da Silva et al. 2008; Saldaña-Vázquez 2014). Similarly, Carollia had a low consumption of Piper and Ficus in this study and a high incidence of Vismia, contrary to previous studies that consider this bat genus as a specialist of Piper and Ficus fruits, which may represent 65 % of its diet (Mikich 2002; López and Vaughan 2007; Gonçalves da Silva et al. 2008; Suárez-Castro and Montenegro 2015). In contrast to previous research (Andrade et al. 2013, Ríos-Blanco and Pérez-Torres 2015), we found a greater diet breadth for Carollia than Artibeus or Sturnira. These findings suggest a high trophic plasticity in the diet of Carollia according to the availability of food resources where this bat genus occurs (Mello et al. 2004). Even though a strong relationship between phylogeny and dietary specialization in frugivorous bats has been established (Giannini and Kalko 2004; Saldaña-Vázquez 2014; Sánchez and Giannini 2018), our results regarding a high diet breadth in Carollia may be related to the inclusion of more than one species of this genus in the diet analysis. Also, possibly indicating a specialization of each Carollia species on different food items when coexisting (York and Billings 2009).
Potential importance of bats to seed dispersal in oil palm landscapes. Because the plants dispersed after the production of oil palm areas will depend on the seed rain within this habitat, the dominant species of frugivorous bat ensemble will determine the role of the seed dispersal in this area (Smith and Knapp 2003; Ríos-Blanco and Pérez-Torres 2015). In this respect, the functional identity of the plants dispersed by the most abundant frugivores have important implications in the future restoration process, particularly so because most of them are pioneer plants.
Considering the restoration process that the government demands after the land oil palm production (Ministerio de Ambiente y Desarrollo Sostenible 2015), seed dispersal mediated by biotic agents is critical for facilitating the seed rain. Some studies highlight the relevance of seed dispersal provided by animals in terms of economic benefits compared to a man-made replacement of this service (Hougner et al. 2006). In this way, given the key role of bats in reforestation or restoration process, it is fundamental to preserve habitats that maintain bat populations such as forest fragments in agricultural landscapes (Burgar et al. 2015). Besides feeding resources, forest fragments provide shelter, feeding sites, roosts, and breeding opportunities for bat species (López and Vaughan 2007; Treitler et al. 2016). Therefore, conserving forest fragments and other similarly important landscape elements (e. g., shrubs, savannas, lagoons, grasslands), may enhance the restoration of agricultural areas, such as oil palm plantations. Although oil palm expansion is a cornerstone in the national agricultural development (Ministerio de Agricultura y Desarrollo Rural 2006), this productive system can be well-adapted to minimize ecological impacts on species (Ocampo-Peñuela et al. 2018). It is crucial to make a balance between the economic and environmental goals in order to produce oil palm with better management practices (Pardo and Campbell 2019).











 nueva página del texto (beta)
nueva página del texto (beta)


