Introduction
Fleas and sucking lice are important vectors of multiple pathogens causing major epidemics worldwide, such as plague (Yersinia pestis) and epidemic typhus (Rickettsia prowazekii). Despite the historical importance of both diseases, this group of ectoparasites has been little studied with respect to other vectors such as mosquitoes or ticks (Gillespie et al. 2009; Bitam et al. 2010; Eisen and Gage 2012). However, these groups of insects are hosts for a wide range of largely understudied pathogens, especially several species of bacteria of the genera Bartonella and Rickettsia (Bitam et al. 2010). The genus Bartonella includes at least 33 species of Gram-negative, intracellular and slow-growing coccobacilli with complex life cycles including multiple vertebrate hosts and vectors, such as B. elizabethae and B. vinsonii arupensis, declared pathogens causing endocarditis in humans and dogs (Breitschwerdt and Kordick 2000; Tsai et al. 2011; Kosoy et al. 2012; Regier et al. 2016). On the other hand, Rickettsia encompasses 26 species of obligate intracellular bacteria which are transmitted by different groups of hematophagous arthropods such as ticks, lice and fleas (Fournier and Raoult 2009; Merhej et al. 2014). Rickettsia species are classified into four groups, two of which are pathogens for man: members of the Spotted Fever group [SGF] (R. conorii, R. massiliae, R. rickettsii and R. parkeri) and Typhus group [TG] (R. prowazekii and R. typhi), this latter group is transmitted exclusively by lice and fleas, which cause epidemic and murine typhus (Fournier et al. 2003; Fournier and Raoult 2009).
In recent decades with the advent of molecular biology techniques, the number of species or strains of both bacteria genera has increased exponentially (Merhej et al. 2014; Regier et al. 2016). Particularly, fleas and sucking lice associated with rodents are the groups in which more studies have focused for the detection of pathogens, with the identification of 16 validated species of Bartonella, nine of Rickettsia and more than 17 new linages near to several validated taxa (but which require isolation for formal identification) for both genera, associated with 45 flea species and seven sucking lice which are also associated with 42 species of rodents in 24 countries around the world (Table 1).
Table 1 Bartonella and Rickettsia species detected in fleas and sucking lice associated with rodents worldwide
| Bacteria species | Flea | Host | Country | References |
|---|---|---|---|---|
| B. birtlesii | Ctenophtalmus andorrensis catalanensis | Apodemus sylvaticus | Spain | Cevidanes et al. 2017 |
| Leptopsylla taschenbergi amitina | A. sylvaticus | Spain | Cevidanes et al. 2017 | |
| B. coopersplainsensis | Stephanocircus pectinipes | Rattus fuscipes | Australia | Kaewmongkol et al. 2011 |
| B. doshiae | Xenopsylla cheopis | Rattus sp. | Afghanistan | Marie et al. 2006 |
| B. elizabethae | Leptopsylla segnis | Mus spretus | Algeria | Bitam et al. 2012 |
| Synosternus cleopatrae | Gerbillus pyramidum | Israel | Morick et al. 2010 | |
| Synopsyllus fonquerniei | Rattus rattus | Madagascar | Brook et al. 2017 | |
| X. cheopis | Rattus norvergicus | Algeria | Bitam et al. 2012 | |
| USA | Frye et al. 2015 | |||
| R. rattus | Algeria | Bitam et al. 2012 | ||
| Rattus tanezumi | Indonesia | Winoto et al. 2005 | ||
| Rattus sp. | Afghanistan | Marie et al. 2006 | ||
| Nigeria | Kamani et al. 2013 | |||
| B. grahamii | Ctenophthalmus agyrtes | ND | Lithuania | Lipatova et al. 2015 |
| Ct. andorrensis catalanensis | A. sylvaticus | Spain | Cevidanes et al. 2017 | |
| Ctenophthalmus nobilis | Myodes glareolus | England | Bown et al. 2004 | |
| Megabothris turbidus | ND | Lithuania | Lipatova et al. 2015 | |
| Megabothris walkeri | ND | Lithuania | Lipatova et al. 2015 | |
| Sy. cleopatrae | ND | Israel | Rzotkiewicz et al. 2015 | |
| Xenopsylla ramesis | ND | Israel | Rzotkiewicz et al. 2015 | |
| B. henselae | X. ramesis | ND | Israel | Rzotkiewicz et al. 2015 |
| Meriones tristrami | Israel | Morick et al. 2010 | ||
| B. koehlerae | Xenopsylla gerbilli | Meriones lybicus | Afghanistan | Marie et al. 2006 |
| B. phoceensis | X. cheopis | R. tanezumi | Indonesia | Winoto et al. 2005 |
| B. queenslandensis | X. cheopis | Rattus sp. | Thailand | Klangthong et al. 2015 |
| B. quintana | X. gerbilli | Meriones lybicus | Afghanistan | Marie et al. 2006 |
| B. rattaustraliani | Stephanocircus dasyure | R. fuscipes | Australia | Kaewmongkol et al. 2011 |
| B. rattimassiliensis | X. cheopis | R. tanezumi | Indonesia | Winoto et al. 2005 |
| B. rochalimae | X. cheopis | R. norvergicus | USA | Frye et al. 2015 |
| B. taylorii | Ct. agyrtes | ND | Lithuania | Lipatova et al. 2015 |
| Ct. andorrensis catalanensis | A. sylvaticus, C. russula, M. spretus |
Spain | Cevidanes et al. 2017 | |
| Ct. nobilis | M. glareolus | England | Bown et al. 2004 | |
| Ctenophthalmus uncinatus | ND | Lithuania | Lipatova et al. 2015 | |
| Hystrichopsylla talpae | ND | Lithuania | Lipatova et al. 2015 | |
| L. taschenbergi amitina | A. sylvaticus | Spain | Cevidanes et al. 2017 | |
| M. turbidus | ND | Lithuania | Lipatova et al. 2015 | |
| M. walkeri | ND | Lithuania | Lipatova et al. 2015 | |
| X. gerbilli | M. lybicus | Afghanistan | Marie et al. 2006 | |
| B. tribocorum | Ctenophtalmus sp. | ND | Nigeria | Kamani et al. 2013 |
| X. cheopis | R. norvergicus | USA | Reeves et al. 2007a; Frye et al. 2015 | |
| R. rattus | Algeria | Bitam et al. 2012 | ||
| R. tanezumi flavipectus | China | Li et al. 2007 | ||
| Rattus sp. | Thailand | Klangthong et al. 2015 | ||
| B. vinsonii | Polygenis bohlsi bohlsi | Thrichomys fosteri | Brazil | de Sousa et al. 2018 |
| Polygenis gwyni | Sigmodon hispidus | USA | Abbot et al. 2007 | |
| B. vinsonii arupensis | Malareus sinomus | Peromyscus eremicus | México | Zapata-Valdés et al. 2018 |
| Orchopeas leucopus | P. eremicus | |||
| Peromyscus leucopus, Peromyscus maniculatus | Fernández-González et al. 2016 | |||
| Pleochaetis exilis | Onycomys torridus | Zapata-Valdés et al. 2018 | ||
| B. vinsonii vinsonii | Ctenophthalmus pseudagyrtes | Microtus sp. | USA | Reeves et al. 2007a |
| Meringis parkeri | Onychomys arenicola, Onychomys leucogaster | México | Fernández-González et al. 2016 | |
| Orchopeas sexdentatus | Neotoma albigula | México | Fernández-González et al. 2016 | |
| Pleochaetis exilis | N. albigula, O. arenicola, O. leucogaster, P. maniculatus | México | Fernández-González et al. 2016 | |
| B. washoensis | Orchopeas hirsuta | Cynomys sp. | USA | Stevenson et al. 2003; Reeves et al. 2007b |
| Cynomys ludovicianus | México | Zapata-Valdés et al. 2018 | ||
| Orchopeas howardi | Sciurus carolinensis | USA | Durden et al. 2004 | |
| Oropsylla montana | Otospermophilus beecheyi | USA | Osikowicz et al. 2016 | |
| Pulex sp. | C. ludovicianus | México | Fernández-González et al. 2016 | |
| Thrassis fotus | Cynomys sp. | USA | Reeves et al. 2007b | |
| Bartonella near birtlesii | O. howardi | S. carolinensis | USA | Reeves et al. 2005b |
| Bartonella near clarridgeiae | Ctenophthalmus lushuiensis | Eothenomys sp. | China | Li et al. 2007 |
| L. segnis | R. rattus | Egypt | Loftis et al. 2006 | |
| P. gwyni | S. hispidus | USA | Abbot et al. 2007 | |
| Bartonella near doshiae | Ct. andorrensis catalanensis | A. sylvaticus | Spain | Cevidanes et al. 2017 |
| L. taschenbergi amitina | A. sylvaticus | Spain | Cevidanes et al. 2017 | |
| Bartonella near elizabethae | Ct. andorrensis catalanensis | A. sylvaticus | Spain | Cevidanes et al. 2017 |
| Leptopsylla algira | ND | Israel | Rzotkiewicz et al. 2015 | |
| Mus musculus | Israel | Morick et al. 2010 | ||
| L. taschenbergi amitina | A. sylvaticus | Spain | Cevidanes et al. 2017 | |
| Ornithophaga sp. | M. spretus | Portugal | De Sousa et al. 2006 | |
| Stenoponia tripectinata | M. spretus | Portugal | De Sousa et al. 2006 | |
| R. rattus | Portugal | De Sousa et al. 2006 | ||
| Sy. cleopatrae | ND | Israel | Rzotkiewicz et al. 2015 | |
| G. pyramidum | Israel | Morick et al. 2010 | ||
| X. cheopis | Rattus sp. | Thailand | Klangthong et al. 2015 | |
| X. ramesis | ND | Israel | Rzotkiewicz et al. 2015 | |
| Bartonella near grahamii | Meringis altipecten | O. arenicola, O. leucogaster, Dipodomys merriami | México | Fernández-González et al. 2016 |
| Meringis arachis | O. arenicola, O. leucogaster, D. merriami | México | Fernández-González et al. 2016 | |
| M. parkeri | O. arenicola, O. leucogaster, D. merriami | México | Fernández-González et al. 2016 | |
| Nosopsyllus fasciatus | Rattus surifer | Thai-Myanmar Border | Parola et al. 2003 | |
| P. exilis | O. arenicola, O. leucogaster | México | Fernández-González et al. 2016 | |
| Sy. cleopatrae | Meriones sacramenti | Israel | Morick et al. 2010 | |
| X. ramesis | ND | Israel | Rzotkiewicz et al. 2015 | |
| Bartonella near henselae | Or. howardi | Glaucomys volans | USA | Reeves et al. 2007a |
| Sy. cleopatrae | Gerbillus andersoni allenbyi | Israel | Morick et al. 2010 | |
| Bartonella near phoceensis | X. cheopis | R. norvergicus, R. rattus | Egypt | Loftis et al. 2006 |
| Bartonella near quintana | Or. howardi | S. carolinensis | USA | Durden et al. 2004 |
| Bartonella near rochalimae | L. taschenbergi amitina | A. sylvaticus | Spain | Cevidanes et al. 2017 |
| X. cheopis | R. norvegicus | Algeria | Bitam et al. 2012 | |
| X. ramesis | ND | Israel | Rzotkiewicz et al. 2015 | |
| Bartonella near taylorii | Ct. lushuiensis | Eothenomys sp. | China | Li et al. 2007 |
| Bartonella near tribocorum | X. cheopis | R. rattus | Benin | Leulmi et al. 2014 |
| Bartonella near vinsonii arupensis | Sy. cleopatrae | ND | Israel | Rzotkiewicz et al. 2015 |
| Bartonella sp. | Echinophaga gallinacea | Dipodomys spectabilis | México | Fernández-González et al. 2016 |
| Ct. andorrensis catalanensis | C. russula | Spain | Cevidanes et al. 2017 | |
| M. arachis | D. spectabilis | México | Fernández-González et al. 2016 | |
| M. altecpin | D. spectabilis, O. arenicola | México | Fernández-González et al. 2016 | |
| Or. hirsuta | Cynomys sp. | USA | Reeves et al. 2007b | |
| Sy. cleopatrae | ND | Israel | Rzotkiewicz et al. 2015 | |
| Thrassis aridis | D. spectabilis | México | Fernández-González et al. 2016 | |
| X. cheopis | R. norvegicus | Algeria | Bitam et al. 2012 | |
| R. rattus | Algeria, Israel | Morick et al. 2010; Bitam et al. 2012 | ||
| R. conorii | Stivalius aporus | Mus caroli | Taiwan | Kuo et al. 2016 |
| R. felis | Acropsylla episema | Apodemus agrarius | Taiwan | Kuo et al. 2016 |
| Anomiopsyllus nudata | N. albigula | USA | Stevenson et al. 2005 | |
| Ctenocephalides felis | Peromyscus yucatanicus | México | Peniche Lara et al. 2015 | |
| R. norvegicus | Cyprus | Psaroulaki et al. 2006 | ||
| R. rattus | Cyprus | Psaroulaki et al. 2006 | ||
| Ct. agyrtes | Apodemus flavicollis | Lithuania | Radzijevskaja et al. 2018 | |
| Ctenophthalmus calceatus calceatus | Lophuromys aquilus | Tanzania | Leulmi et al. 2014 | |
| Ctenophtalmus sp. | R. norvegicus | Portugal | De Sousa et al. 2006 | |
| H. talpae | Micromys minutus | Lithuania | Radzijevskaja et al. 2018 | |
| L. segnis | Mus sp. | Algeria | Bitam et al. 2009 | |
| Polygenis odiosus | Ototylomys phyllotis | México | Peniche Lara et al. 2015 | |
| S. aporus | M. caroli | Taiwan | Kuo et al. 2016 | |
| X. cheopis | R. norvegicus | Cyprus | Christou et al. 2010 | |
| R. rattus | Cyprus, Madagascar | Christou et al. 2010; Rakotonanahary et al. 2017 | ||
| Rattus sp. | Afghanistan, Algeria | Marie et al. 2006; Bitam et al. 2009 | ||
| R. helvetica | Ct. agyrtes | A. flavicollis | Lithuania | Radzijevskaja et al. 2018 |
| M. turbidus | A. flavicollis | |||
| M. minutus | ||||
| M. walkeri | A. flavicollis | |||
| R. japonica | S. aporus | M. caroli | Taiwan | Kuo et al. 2016 |
| R. monacensis | Ct. agyrtes | A. flavicollis | Lithuania | Radzijevskaja et al. 2018 |
| R. raoultii | ND | A. flavicollis, Myodes glareolus | Germany | Obiegala et al. 2016 |
| R. typhi | Ctenophthalmus congeneroides | A. agrarius | South Korea | Kim et al. 2010 |
| L. segnis | R. norvegicus | Cyprus | Christou et al. 2010 | |
| R. rattus | Cyprus, Egypt, Portugal | De Sousa et al. 2006, Loftis et al. 2006; Christou et al. 2010 | ||
| Rhadinopsylla insolita | A. agrarius | South Korea | Kim et al.2010 | |
| Xenopsylla brasiliensis | Mastomys natalensis | Tanzania | Leulmi et al. 2014 | |
| R. rattus | Tanzania | Leulmi et al. 2014 | ||
| Rattus sp. | Democratic Republic of the Congo | Leulmi et al. 2014 | ||
| X. cheopis | R. norvegicus | Cyprus, Egypt | Loftis et al. 2006; Christou et al. 2010 | |
| R. rattus | Benin, Cyprus, Egypt, Madagascar | Loftis et al. 2006; Christou et al. 2010; Leulmi et al. 2014, Rakotonanahary et al. 2017 | ||
| Rattus sp. | Argelia | Bitam et al. 2009 | ||
| Rickettsia prowazekii | Or. howardii | G. volans | USA | Sonenshine et al. 1978 |
| Candidatus Rickettsia Asemboensis | E. gallinacea | R. rattus | Egypt | Loftis et al. 2006 |
| S. cleopatrae | ND | Israel | Rzotkiewicz et al. 2015 | |
| X. ramesis | Gerbillus dasyurus, Meriones tristrami, M. musculus | Israel | Rzotkiewicz et al. 2015 | |
| Rickettsia felis-like | X. ramesis | ND | Israel | Rzotkiewicz et al. 2015 |
| Rickettsia near monacensis | Oropsylla hirsuta | Cynomys sp. | USA | Reeves et al. 2007b |
| Rickettsia sp. Oh16 | Or. howardi | S. carolinensis | USA | Reeves et al. 2005 |
| Rickettsia sp. TwKM01 | S. aporus | A. agrarius | Taiwan | Kuo et al. 2016 |
| Rickettsia endosymbiont of Eucoryphus brunneri | Ct. agyrtes | A. flavicollis | Lithuania | Radzijevskaja et al. 2018 |
| B. henselae | Neohaematopinus sciuri | S. carolinensis | USA | Durden et al. 2004 |
| B. phoceensis | Hoplopleura pacifica | R. norvegicus | Egypt | Reeves et al. 2006 |
| Polyplax spinulosa | R. norvegicus | Taiwan | Tsai et al. 2010 | |
| Polyplax sp. | R. rattus | Madagascar | Brook et al. 2017 | |
| Rattus sp. | Thailand | Klangthong et al. 2015 | ||
| B. rattimassiliensis | Hoplopleura pacifica | R. norvegicus | Egypt | Reeves et al. 2006 |
| Polyplax spinulosa | R. norvegicus | Egypt, Taiwan | Reeves et. al. 2006; Tsai et al. 2010 | |
| Polyplax sp. | R. rattus | Madagascar | Brook et al. 2017 | |
| Rattus sp. | Thailand | Klangthong et al. 2015 | ||
| B. tribocorum | Polyplax spinulosa | R. norvegicus | Taiwan | Tsai et al. 2010 |
| B. vinsonii | Hoplopleura hirsuta | S. hispidus | México | Sánchez-Montes et al. 2016b |
| B. washoensis | Neohaematopinus sciuri | S. carolinensis | USA | Durden et al. 2004 |
| Bartonella near tribocorum | Polyplax spinulosa | R. norvegicus | Egypt | Reeves et al. 2006 |
| Bartonella near washoensis | Hoplopleura sciuricola | S. carolinensis | USA | Durden et al. 2004 |
| Bartonella sp. | Polyplax sp. | Thrichomys apereoides | Brazil | Fontalvo et al. 2017 |
| R. prowazekii | Neohaematopinus sciuropteri | G. volans | USA | Sonenshine et al. 1978 |
| Polyplax spinulosa* | R. norvegicus | México | Mooser et al. 1931 | |
| R. typhi | Enderleinellus marmotae | Marmota monax | USA | Reeves et al. 2005 |
| Hoplopleura pacifica | R. norvegicus | Egypt | Reeves et al. 2006 |
In México, nine taxa of fleas (Ctenocephalides felis, Maleareus sinomus, Meringis parkeri, Orchopeas hirsuta, O. leucopus, O. sexdentatus, Pleochaetis exilis, Pulex sp., and Polygenis odiosus) and two species of sucking lice (Hoplopleura hirsuta and Polyplax spinulosa) tested positive for at least one of four validated species of Bartonella (B. vinsonii and B. washoensis) and Rickettsia (R. felis and R. prowazekii). Additionally new lineages of Bartonella have been registered in six more flea species (Echinophaga gallinacea, Meringis altipecten, M. arachis, M. parkeri, Pleochaetis exilis, Thrassis aridis, Table 1). These records came from isolated studies carried out in wildlife from the southeast and northern parts, lacking data regarding central México where there is a report of human cases of murine typhus (Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades 2018; Sánchez-Montes et al. 2019). Additionally, for México, 172 species of fleas and 44 species of sucking lice, have been recorded, then, the inventory of species of both bacteria genera is still far from complete (Sánchez-Montes et al. 2013; Acosta-Gutiérrez 2014).
Due to the great diversity of potential vectors and the historical presence of human cases of murine typhus in the centre of the country; the purpose of this study was to identify the presence and diversity of Bartonella and Rickettsia species in a focus of murine typhus in Hidalgo, México.
Material and Methods
During August to September 2014, we sampled in two private ranches from Mineral del Monte and Tulancingo de Bravo (Figure 1), in the state of Hidalgo, México, close to sites where human murine typhus cases have been reported (CENAPRECE 2016). This study was approved by the Ethics and Research Committee of the Medical Faculty of the Universidad Nacional Autónoma de México [FMED/CI/JMO/004/2012].
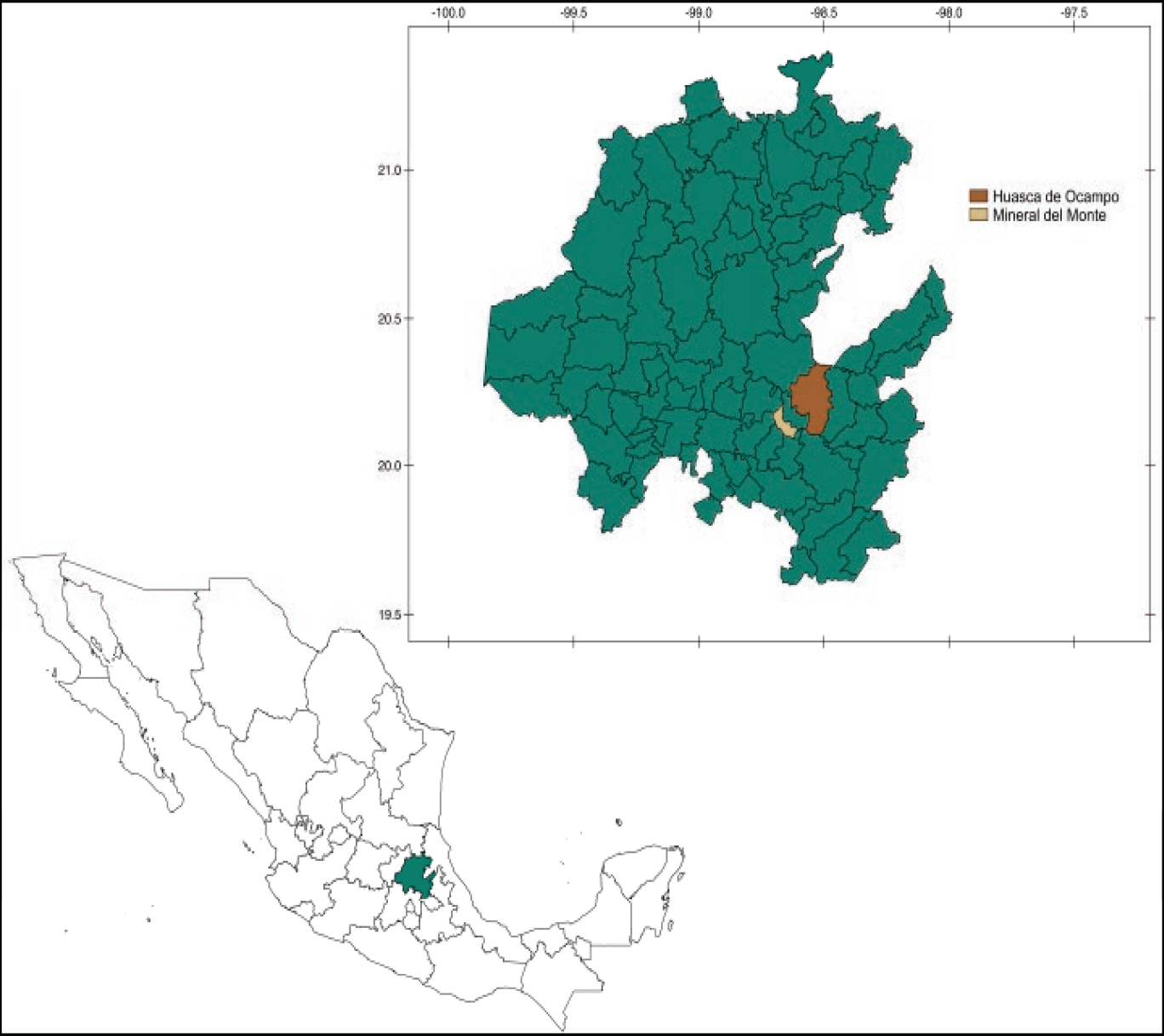
Figure 1 Sampling sites along the state of Hidalgo, México. Green: State of Hidalgo; Brown: Huasca de Ocampo; Yellow: Mineral del Monte.
In order to identify the presence of several flea-borne and louse-borne pathogens (Rickettsia and Bartonella) in small mammals and their associated ectoparasites, we trapped small mammals using Sherman traps following Romero-Almaraz et al. (2007), under permission FAUT-0170 from the Secretaría del Medio Ambiente y Recursos Naturales. All mammals were sacrificed in accordance with the Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research (Sikes et al. 2016). We performed the necropsy of each animal, extracting a portion of liver and ear which were fixed in 96 % ethanol until its processing in the laboratory. Additionally, fleas and lice were recovered from host’s bodies by manual inspection and fixed in absolute ethanol. Hosts and fleas were identified and deposited at the Mammal Collection and the Flea Collection of the Museo de Zoología “Alfonso L. Herrera” Facultad de Ciencias (MZFC) and Colección del Centro de Medicina Tropical, Facultad de Medicina (CMTFM), both belonging to Universidad Nacional Autónoma de México.
For morphological determination, fleas and lice were mounted on slides using the modified techniques of Kim et al. (1986) and Wirth and Marston (1968). Species were identified using specialized taxonomic keys such as Kim et al. (1986) for lice and Acosta and Morrone (2003), Hastriter (2004), Hopkins and Rothschild (1971), Morrone et al. (2000), and Traub (1950) for fleas.
From collected ectoparasites and hosts tissues, we extracted DNA with the QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany). As an endogenous internal control and for molecular identification of the ectoparasites, we amplified a fragment of 400 bp of Cytochrome Oxidase Subunit I (COI) gene. For pathogens detection, we amplified a fragment of gltA and ompB genes specific for each group using primers and temperature conditions previously reported (Table 2).
Table 2 Oligonucleotide primers used in this study.
| Gen | Primers | Sequence (5´-3´) | Length (bp) | Reference |
|---|---|---|---|---|
| Fleas and lice | ||||
| COI (Cytochrome oxidase subunit I) | L6625 | CCGGATCCTTYTGRTTYTTYGGNCAYCC | 400 | Hafner et al. 1994 |
| H7005 | CCGGATCCACNACRTARTANGTRTCRTG | |||
| Rickettsia sp. | ||||
| gltA (Citrate synthase) | RpCS.415 | GCTATTATGCTTGCGGCTGT | 806 | de Souza et al. (2006) |
| RpCS.1220 | TGCATTTCTTTCCATTGTGC | |||
| ompB (Outer membrane protein B) | 120-M59 | CCGCAGGGTTGGTAACTGC | 862 | Roux and Raoult, 2000 |
| 120-807 | CCTTTTAGATTACCGCCTAA | |||
| Bartonella sp. | ||||
| gltA (Citrate synthase) | BhCS781.p | GGGGACCAGCTCATGGTGG | 379 | Norman et al. 1995 |
| BhCS1137.n | AATGCAAAAAGAACAGTAAACA | |||
The reaction mixture consisted of 12.5 μL of GoTaq® Green Master Mix, 2X of Promega Corporation (Madison, WI, USA), the pair of primers (100 ng each), 6.5 μL nuclease-free water and 30 ng DNA in a final volume of 25 μL (Sánchez-Montes et al. 2016a, b).
PCR products were resolved in 2 % agarose gels using TAE buffer at 85 V during 45 minutes and visualized using an ODYSSEY CLx Imaging System (LICOR Biosciences). Purified amplification products were submitted for sequencing at Macrogen Inc., Korea.
Sequences were analysed and edited using Bioedit version 5.0.9 Sequencing Alignment Editor Copyright © program and deposited in GenBank under accession numbers (MG952757 to MG952772). In order to identify the species of Bartonella and Rickettsia, we used the similarity criteria of the gltA and ompB genes proposed by La Scola (2003), Fournier and Roult (2009) and Fournier et al. (2003). Global alignments were done using Clustal W (Thompson et al. 1994) and the best substitution model was selected based on the lowest BIC (Bayesian Information Criterion) score for each gene using MEGA 6.0 (Tamura et al. 2011; Sánchez-Montes et al. 2016c). Additionally phylogenetic reconstruction was done using Maximum Likelihood also in MEGA 6.0 and branch support was evaluated over 10,000 bootstrap replications.
Results
We collected 40 rodents from four species (Mus musculus, Peromyscus beatae, Rattus norvergicus, and Reithrodontomys sumichrasti), and one shrew (Sorex ventralis), which are deposited in the MZFC under the following catalogue numbers LRR001 to LRR040. We detected the presence of Bartonella DNA in four samples of liver of two P. beatae (2/26 = 7.69 %) and two R. norvergicus (2/4 =50 %). Sequences recovered from P. beatae exhibited a similarity of 98 % with B. vinsonii vinsonii (a member of the Bartonella vinsonii complex) and those from R. norvergicus corresponded in a 100 %, respectively with B. elizabethae (Figure 2). In the case of Rickettsia detection, a single specimen of R. norvergicus (1/4 = 25 %) tested positive in samples from liver and ear; we recovered sequences of gltA and ompB genes which exhibited a similarity of 99 % and 100 % with R. typhi (Accesion number AE017197) deposited in GenBank (Figure 3). A single R. norvergicus specimen presents co-infection between B. elizabethae and R. typhi.
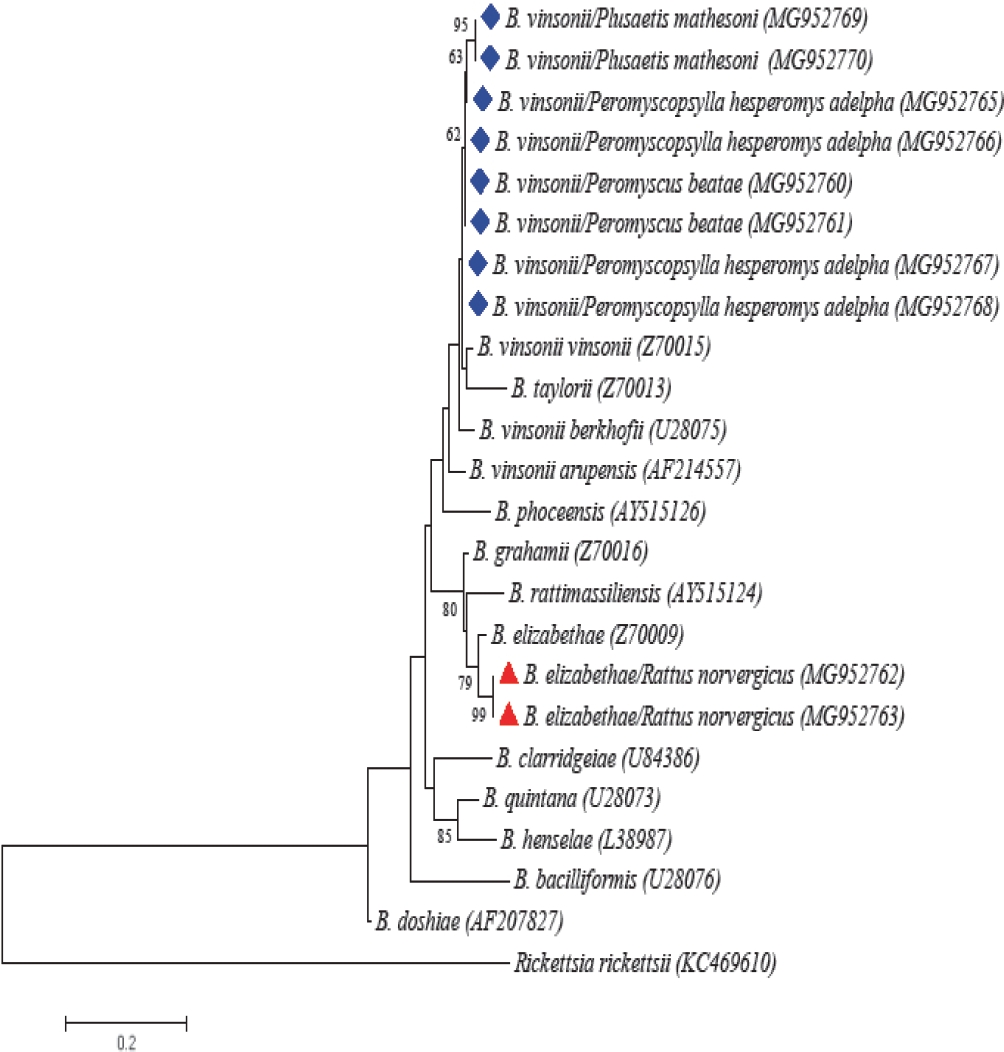
Figure 2 Maximum likelihood (ML) phylogenetic tree generated with gltA gene (300 bp) from several members of the genus Bartonella. The nucleotide substitution model was the Tamura three parameter model (T92) with discrete Gamma distribution (+G). Bootstrap values higher than 50 are indicated at the nodes. Sequences recovered in the study are marked with blue rhombuses and red triangles.
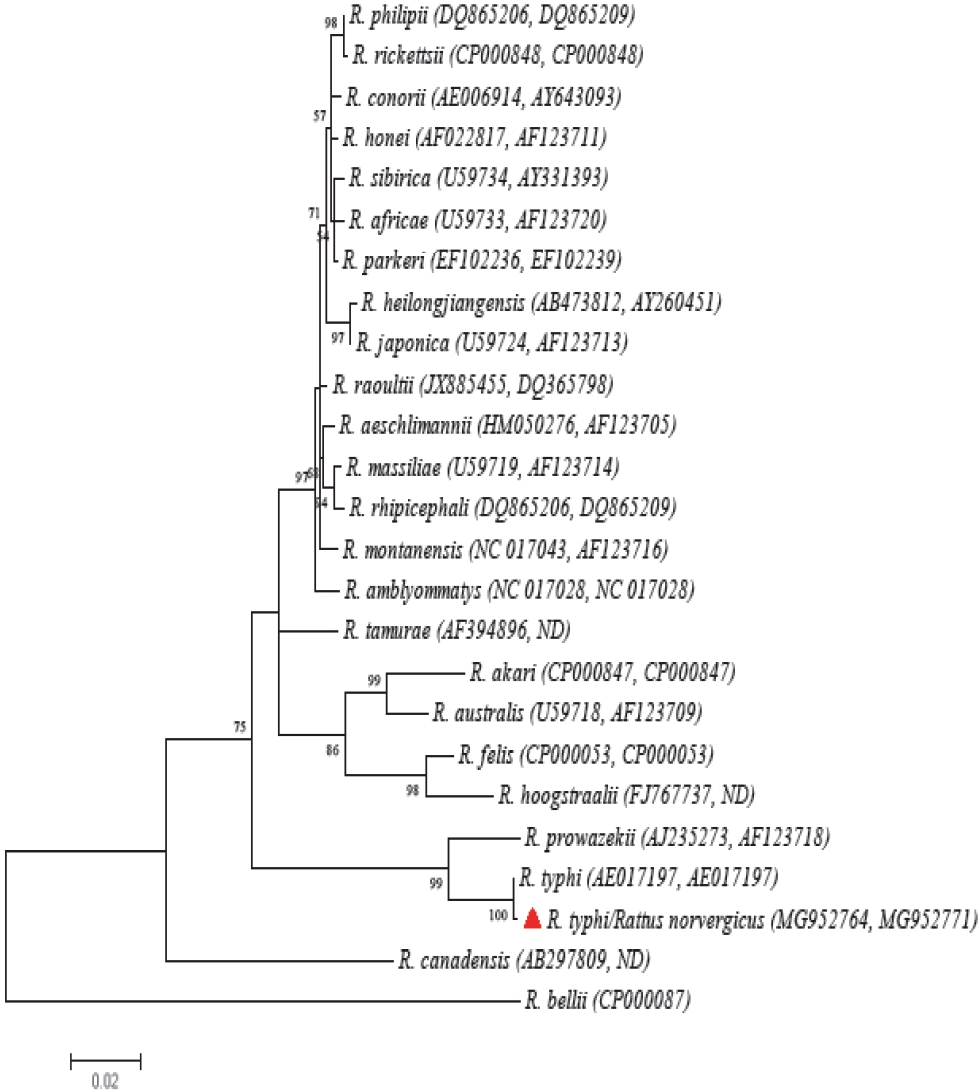
Figure 3 Maximum likelihood (ML) phylogenetic tree generated with gltA and ompB genes concatenated (1547 bp) from several members of the genus Rickettsia. The nucleotide substitution model was the Tamura three parameter model (T92) with discrete Gamma distribution (+G). Bootstrap values higher than 50 are indicated at the nodes. Sequences recovered in the study are marked with red triangles.
Hosts were infested by 47 fleas (18 females, 29 males), and 172 sucking lice (60 females, 39 males, 73 nymphs), distributed in six taxa, five species belonging to five families and six genera (Table 3). No fleas or lice were recovered from M. musculus and S. ventralis. After morphological identification was done, we amplified a fragment of 400 bp of Cytochrome oxidase subunit I (COI) in all ectoparasites recovered, in order to corroborate the identification of all samples, especially of those damaged specimens and nymphal stages. DNA sequences of the COI for four of the six species analysed were deposited in GenBank with the following accession numbers: C. tecpin (MG952757), P. hesperomys adelpha (MG952758); P. mathesoni (MG952759), P. spinulosa (MG952772) and H. reithrodontomydis (KT151126). No complete sequences were obtained for J. b. breviloba. We detected the presence of the same Bartonella lineage previously refereed in P. beatae, in two flea species (six P. hesperomys adelpha and 17 P. mathesoni) recovered from the two hosts which tested positive and from three others that were negative (Table 3). Sequences from fleas and hosts shape a single cluster within our phylogenetic analysis (Fig. 1). None of the flea or sucking lice species analysed was positive for Rickettsia DNA.
Table 3 Ecological parameters of Bartonella and Rickettsia species detected in fleas, sucking lice and small mammals in Hidalgo, México.
| Host | Ectoparasite | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Species | n | HI | % | BAD | Family | Species | HP | EA | % | A | II | EI | % | BAD |
| Ranch 1 Tulancingo de Bravo | |||||||||||||||
| Cricetidae | Peromyscus beatae | 20 | 2 | 10 | Bartonella vinsonii | Ceratophyllidae | Jellisonia breviloba breviloba | 2 | 3 | 10 | 0 | 2 | 0 | 0 | ND |
| Plusaetis mathesoni | 10 | 27 | 5 | 1 | 3 | 17 | 57 | Bartonella vinsonii | |||||||
| Ctenophtalmidae | Ctenophtalmus tecpin | 2 | 3 | 10 | 0 | 2 | 0 | 0 | ND | ||||||
| Leptopsyllidae | Peromyscopsylla hesperomys adelpha | 4 | 7 | 20 | 0 | 2 | 6 | 86 | Bartonella vinsonii | ||||||
| Reithrodontomys sumichrasti | 2 | 0 | 0 | ND | Hoplopleuridae | Hoplopleura reithrodontomydis | 1 | 4 | 50 | 2 | 4 | 0 | 0 | ND | |
| Soricidae | Sorex ventralis | 1 | 0 | 0 | ND | NR | NR | 0 | NR | (-) | (-) | (-) | NR | NR | ND |
| Ranch 2 Mineral del Monte | |||||||||||||||
| Cricetidae | Peromyscus beatae | 6 | 0 | 0 | ND | Ceratophyllidae | Plusaetis mathesoni | 1 | 3 | 17 | 1 | 3 | 0 | 0 | ND |
| Muridae | Mus musculus | 8 | 0 | 0 | ND | NR | NR | 0 | NR | (-) | (-) | (-) | NR | NR | ND |
| Rattus norvergicus | 4 | 2 | 50 | Bartonella elizabethae | Polyplacidae | Polyplax spinulosa | 4 | 172 | 100 | 43 | 43 | 0 | 0 | ND | |
| 1 | 25 | Rickettsia typhi | |||||||||||||
n: Host collected; HI: Number of hosts infected; %: Prevalence; BAD: Bacterial agents detected; HP: Host parasitized; EA: Ectoparasites collected; A: Mean abundance; II: Intensity of infestation; EI: Ectoparasites infected; NR: Not recovered; ND: Not detected.
Discussion
We report for the first time the presence of two species of Bartonella and one of Rickettsia in the state of Hidalgo, México. The first Bartonella species is a member of the B. vinsonii complex, closely related with previous sequences detected in Cricetid rodents and fleas of the northern México (Rubio et al. 2014; Fernández-González et al. 2016). Also, this is the first study to report the presence of a Bartonella in the fleas P. hesperomys adelpha and P. mathesoni and in the host P. beatae (Table 1). Our phylogenetic analysis grouped sequences of B. vinsonii from P. hesperomys adelpha, P. mathesoni and P. beatae in a single cluster, then, our inference is that both flea species could be the potential vectors of these. Additionally, positive P. hesperomys adelpha were recovered from negative hosts, suggesting that these fleas may disseminate the pathogen in non-infected individuals among the rodent population bacteria (Kosoy et al. 1997; Morick et al. 2010). However, it is necessary to carry out tests to verify their vectorial capacity. Both species of fleas have a restricted distribution in México, which extend along the northeastern and central parts of the country, parasitizing several cricetid species such as Peromyscus levipes, P. maniculatus, Reithrodontomys megalotis (P. mathesoni) and P. difficilis (P. hesperomys adelpha), so it is not unexpected that this strain of bacteria is widely distributed in the country (Ponce-Ulloa and Llorente-Bousquets 1993; Hoffman et al. 1989; Whitaker and Morales-Malacara 2005; Acosta and Fernández 2015).
We also report for the first time the presence of B. elizabethae in México, a zoonotic bacterial that may causes endocarditis and neuroretinitis in humans. This agent was reported for the USA in the 1990’s, however, is has become an emerging problem in several countries of Southeast Asia, Portugal and France (Regier et al. 2016; Tay et al. 2016). Bartonella elizabethae is mainly transmitted by the rat flea Xenosylla cheopis (Table 1); however, in our study we did not recovered any fleas from the four R. norvergicus analysed. The higher prevalence of B. elizabethae in collected murid rodents suggests the presence of this flea or other competent vector in the area (Bitam et al. 2012). Additionally, we compiled evidence for the first time of the presence of R. typhi in rodents of the state of Hidalgo. This Rickettsia produces febrile cases with a wide range of severity that can lead to systemic failure in less than 5% percent of cases (Zavala-Castro et al. 2009). In the state of Hidalgo, three cases of murine typhus had been reported between 2005 to 2010, nevertheless, in 2015 there was an outbreak with 12 cases (Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades 2018).
Only one rat reported coinfection by B. elizabethae and R. typhi, a phenomenon that has been previously reported, probably because both pathogens are transmitted by the same flea species (Table 1). This reinforces the hypothesis of the presence of this vector in the study area (Marie et al. 2006; Bitam et al. 2012; Frye et al. 2015). The presence of positive Norway rats for these two zoonotic pathogens is a risk to human health, because this rodent species invade suburban and urban areas, live and thrive in human settlements and could carry fleas that can feed on human hosts and produce urban outbreaks. Our findings represent the first record of several confirmed zoonotic pathogens that can cause murine typhus and endocarditis in México, which highlight the importance of the establishment of active entomological surveillance in wildlife.











 nueva página del texto (beta)
nueva página del texto (beta)


