Introduction
The puma (Puma concolor) is the felid with the largest distribution in America, occupying areas from southern Canada to southern Chile and Argentina (Currier 1983). In this large range, the species inhabits a wide variety of habitats (i. e., savannas, humid forests, deserts) including some peri-urban landscapes surrounding large cities (Mazzolli 2012; Arias-Alzate et al. 2015). In the Neotropics the species is considered the main predator in mountain ecosystems, especially in areas above 2,000 masl (De Angelo et al. 2009, Arias-Alzate et al. 2015).
Despite its importance as a top predator in most ecosystems that it inhabits (e. g., regulates and maintain prey populations; Hoogesteijn and Hoogesteijn 2005, Hunter and Barrett 2011), most studies about puma diet come from high latitudes or Neotropical lowlands where diet has been reported as highly diverse and strongly dependent on mammals (Chinchilla 1997; Pessino et al. 2001; Moreno et al. 2006; Hernández 2008, Skewes et al. 2012). For instance, diet in North American populations is mostly composed of large ungulates (e. g., Odocoileus hemionus; Iriarte et al. 1990), while in Central America and southern South America the puma shows a tendency towards feeding on medium and small species (e. g., Dasyprocta spp., Cuniculus spp., Pecari tajacu and Mazama spp.; Currier 1983; Emmons 1987; Nuñez et al. 2000; Pessino et al. 2001; Polisar et al. 2003; Novack et al. 2005; Hernández 2008; Pontes and Chivers 2007). In contrast to the work done elsewhere, studies on puma diet in other regions of South America (e. g., northwest) are still scarce, especially in highlands, which have only recently received a little attention (Hernández-Guzmán et al. 2011).
In Colombia, little is known about the foraging ecology of this species and most information comes from opportunistic unpublished records and personal communications with only one systematic study, from Puracé National Natural Park in the central range of the Colombian Andes (departments of Cauca and Huila; Hernández-Guzmán et al. 2011). Here we assess puma diet in the northern Andes, in the highlands of Tamá NNP (National Natural Park; department of Norte de Santander). We emphasize that this knowledge is of great significance, since it provides greater understanding of the species’ ecology and its interactions with other species, information needed for the definition of better strategies for puma management and conservation, especially in a protected area surrounded by human activities.
Materials and methods
Study area. The study was conducted in Tamá NNP, in the southeastern portion of the department Norte de Santander, in the eastern Andean range of Colombia. The park is under jurisdiction of the Toledo and Herrán municipalities between 2,067 and 3,356 masl (7° 02ʼ and 7° 27ʼ N; -72° 02ʼ and -72° 28ʼ W; Figure 1; Cáceres-Martínez et al. 2016). The park covers approximately 480 km2 of the Tamá Massif on the limits with Venezuela; Tamá NNP is part of a bi-national border protected area with El Tamá National Park in Venezuela.

Figure 1 Cover types and locations of collected puma scats within Tamá National Natural Park and its buffer area in the high Andean region. 1. La Carpa. 2. La Rochela. 3. Orocué. 4. Páramo de la Cabrera. 5. Paramo of Tamá. 6. Belchite. 7. Asiria de Belén. 8. Páramo of Santa Isabel. 9. Santa Isabel.
Selection of sampling sites. The puma is the second largest cat present in Tamá NNP with a strict carnivorous diet, after the jaguar (Panthera onca; Cáceres-Martínez et al. 2016), so there is a potential for confusing scats from these two species, mainly when there are not associated tracks or other signs that allow certain species identification. Therefore, to avoid scat misidentification, we conducted the study only at high elevations (2,067 to 3,500 masl) where the jaguar is usually absent (De la Torre et al. 2018); jaguar have been recorded in the park only in lowlands (between 350 and 800 masl; Cáceres-Martínez et al. 2016). In this altitudinal range we selected nine localities for field sampling: La Carpa, La Rochela (buffer zone), Orocué, Páramo of Cabrera, Páramo of Tamá, Belchite, Asiria de Belén, Páramo of Santa Isabel, and Santa Isabel, covering a significant portion of the highlands of the park and buffer zone (Figure 1). Average temperature in these areas ranges from 6 to 16 °C with precipitation variating between 800 and 2,000 mm/year with a frequent mist contributing to the constant humidity of the study area (Meneses et al. 2004).
Collection of scat samples. Scat collection was carried out from line-transects along trails, across natural covers following mountain ridges and on streambanks. At least three transects at each location, from 2 to 3 km in length, were sampled monthly between June 2012 and May 2015. Scats were identified as from puma by the presence of tracks and specific scat characteristics (i. e., diameter, length, presence of hairs), following the morphological description and biometrics of Aranda (2012). Scats were collected and stored in plastic bags for later analysis. For each sample we recorded geographical position, date and elevation.
Food habits and prey biomass analysis. To determine the puma’s diet each scat was measured, weighed, and washed over wire mesh with tap water to separate the components (i. e., hair, bones, claws, and teeth). Afterwards, all samples were dried at room temperature for 48 hours (Ackerman et al. 1984). Each item was identified to species level using a reference collection of mammal specimens from the Instituto de Ciencias Naturales (ICN) collection at the National University of Colombia (Bogotá) and through literature review. All samples were deposited at the mammal collection of the Jose Celestino Mutis museum, Universidad of Pamplona. We also estimated the minimum number of scats required for an adequate diet description through a prey-species accumulation curve (Foster et al. 2010; Hernández-Guzmán et al. 2011). We performed this analysis by randomly adding all samples and estimating average and standard deviations from 1,000 permutations (Gotelli and Colwell 2001), thus eliminating the influence of the order in which each scat was added. The analysis was performed using Vegan Package v 2. 4 - 2 with the function specaccum (Oksanen et al. 2007) implemented in R. We also estimated prey absolute frequency (AF) as the number of times a prey type was present in all scats collected (Rueda-Zozaya 2010) and then estimated the frequency of occurrence (FO) using the ratio of the absolute frequency (AF) to the total number of scats (Rueda-Zozaya 2010). We then calculated the percentage of occurrence (PO) as (PO) = (AF) / the total number of scats x 100.
Biomass consumed (i. e., percentage of biomass) was calculated as the mean weight of each prey species multiplied by the frequency of each species, divided by the total weight of all species in the total scat samples, then multiplied by 100 (Medina et al. 2009). Body weights were based on literature reports (e. g.,Ackerman et al. 1984; Eisenberg 1989; Ramírez 2011). As large-sized prey species are more likely to occur in smaller proportion in the scats than small species, due to differential ingestion and digestion, potentially leading to overestimation of some species (Rueda-Zozaya 2010), we applied a biomass correction factor using Ackerman’s (1984) equation (Y = 1.98 + 0.035X), where Y is the weight of prey consumed per scat and X is the mean weight of the prey species. Prey species identified in scats were corroborated with camera-traps records obtained in the same localities (Cáceres-Martínez et al. 2016).
Results
We collected a total of 45 scat samples (Appendix 1). The accumulation curve approached the asymptote, indicating that most potential prey species were detected within approximately 40 scat samples (Figure 2).

Figure 2 Prey species accumulation curves indicating the number of scats samples required to describe the food habits of puma in the study area; gray shading indicates 95% confidence interval.
We identified eight prey species from the scats; other vertebrate items were found but their species identification was uncertain (Appendix 1) so they were excluded from analysis. The most commonly represented species were the South American coati (Nasua nasua), followed by the dwarf red brocket deer (Mazama rufina) and mountain paca (Cuniculus taczanowskii) with AF values of 9, 6 and 5 respectively (Table 1). Meanwhile, the western mountain coati (Nasuella olivacea), the Central American agouti (Dasyprocta punctata), the nine-banded armadillo (Dasypus novemcinctus) and the two-toed sloth (Choloepus hoffmanni) were less frequently detected (Table 1). The species with the largest FO and PO was N. nasua followed by M. rufina (Table 1).
Table 1 Composition of puma’s diet and relative biomass consumed based on 45 scats collected in Tamá NNP: Absolute frequency (AF), frequency of occurrence (FO), percentage of occurrence (PO), mean prey weight (MPW), biomass correction factor (BCF) and relative biomass consumed (RBC).
| Species prey | FA | FO | PO (%) |
MPW (kg) |
BCF (kg) |
RBC (kg) |
Biomass (%) |
|---|---|---|---|---|---|---|---|
| Nasua nasua | 9 | 0.20 | 20.00 | 3.07 | 2.08 | 18.79 | 28.61 |
| Mazama rufina | 6 | 0.13 | 13.33 | 8.20 | 2.26 | 13.60 | 20.72 |
| Cuniculus taczanowskii | 5 | 0.11 | 11.00 | 13.00 | 2.43 | 12.18 | 18.54 |
| Nasuella olivacea | 3 | 0.06 | 6.00 | 1.50 | 2.03 | 6.10 | 9.29 |
| Choloepus hoffmanni | 2 | 0.04 | 4.40 | 5.70 | 2.18 | 4.36 | 6.64 |
| Didelphis marsupialis | 2 | 0.04 | 4.40 | 5.50 | 2.17 | 4.35 | 6.62 |
| Dasypus novemcinctus | 2 | 0.04 | 4.40 | 3.10 | 2.08 | 4.18 | 6.36 |
| Dasyprocta punctata | 1 | 0.02 | 2.20 | 4.00 | 2.12 | 2.12 | 3.23 |
In terms of biomass consumed, C. taczanowskii presented the greatest contribution per individual (2.435 kg; Table 1); however, N. nasua contributed more to the total biomass consumed (28.6 % of biomass consumed), followed by M. rufina (20.71 %) and C. taczanowskii (18.54 %), together accounting for 67.87 % of the total biomass. The other five species contributed together the remaining 32.13 % of the total biomass consumed by puma (Table 1). No large or domestic species were recorded as consumed by puma in the study area.
Discussion
Our work represents the first study on P. concolor conducted in northeastern Colombia and the second study in a protected area of the Colombian Andes. The minimum number of scats necessary to assess puma diet could vary depending on the conditions of each location, which would require different sampling efforts according to particular temporal and spatial contexts. Our data showed that the minimum number of scats necessary for estimating the species’ diet in our study area is between 31 and 41 scats, suggesting that our sampling (n = 45) was adequate to give an accurate estimate of the species’ diet. This result corresponds with previous studies in Mexico by Monroy-Vilchis et al. (2009) and Núñez et al. (2000) which suggested a minimum of 15 and 40 scats, respectively; in Colombia, a study in Puracé NNP in the Central Andes of Colombia estimated a minimum requisite of 20 scats (Hernández-Guzmán et al. 2011), which overlaps our estimation.
Even though it appears we had a sufficient sampling effort, it is likely some prey species were not detected given our method based on comparisons with reference material (Klare et al. 2011). For instance, 50 % of the samples had unidentified items due to the advanced state of decomposition. To avoid this loss of information, Foster et al. (2010) suggested from species accumulation curves that the minimum sample size required to fully characterize the species’ diet should include at least 100 scats; however, high decomposition rates associated with warm and/or humid conditions, such as those in tropical and cloud forests, may limit the availability and processing of scats.
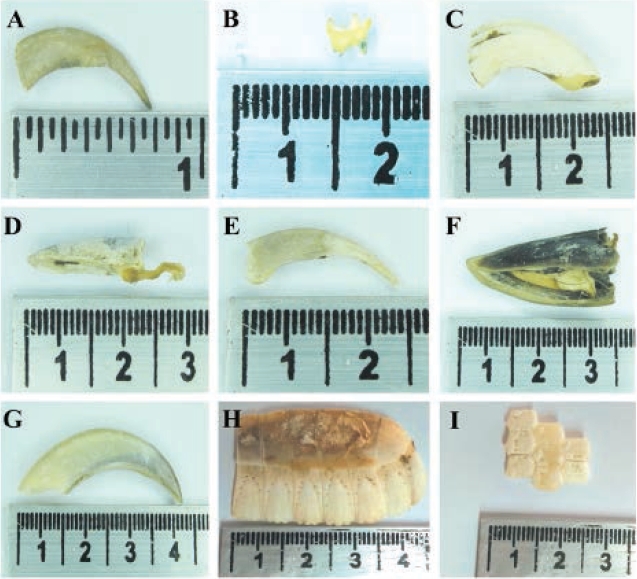
Figure 3 Prey species recorded in puma scat in Tamá NNP a) Nasua nasua, b) Didelphis marsupialis, c) Cuniculus taczanowskii, d) Dasyprocta punctata, e) Nasuella olivacea, f) Mazama rufina, g) Choloepus hoffmanni, h) and i). Dasypus novemcinctus.
Our results showed that the most frequently consumed prey species in the study area are small- to medium-sized mammals. Prey species composition in our results were very similar to those reported in other studies along different areas of the Neotropics (Nuñez et al. 2000, Crawshaw and Quigley 2002, Novack et al. 2005, Moreno et al. 2006, De Azevedo 2008, Foster et al. 2010). However, frequency of species occurrence varies considerably across studies. We found that for Tamá NNP, N. nasua, M. rufina, and C. taczanowskii (followed by N. olivacea) were the most frequently consumed species by puma, which is very similar to previous studies in Central and South America. For instance, numerous studies have found Nasua spp. among the most frequent prey in puma’s diet in México (Nuñez et al. 2000), Belize (Foster et al. 2010), Guatemala (Novack et al. 2005), Costa Rica (Chinchilla 1997), and Brazil (De Azevedo 2008); Mazama spp. have also been among the most frequently reported prey from Belize (Foster et al. 2010), Guatemala (Novack et al. 2005), Costa Rica (Chinchilla 1997) and Brazil (Crawshaw and Quigley 2002, De Azevedo 2008). As in our study, other studies have frequently identified medium-sized rodents (Dasyprocta spp. and Cuniculus spp.) species in the diet, with examples from Panama (Moreno et al. 2006), Bolivia (Pacheco 2004), and Brazil (De Azevedo 2008). Overall, with slight taxonomic differences and some frequency variation, puma’s diet in our study followed patterns similar to that in other regions and ecosystems in the Neotropics.
We found very similar results to those from the only other study on puma’s diet in Colombia (Puracé NNP; Hernández-Guzmán et al. 2011), likely because of the highly similar ecosystems sampled. In Puracé NNP the most frequent prey species found were northern pudú (Pudu mephistophiles), N. olivacea, and Mazama sp. (likely M. rufina). Differences in species composition may reflect that Northern Pudú is not present in the Eastern Andean range of Colombia (Barrio and Tirira 2008) and N. nasua is not abundant above 3,000 masl (González-Maya et al. 2015); however, Mazama sp. was also among the most frequent prey species in Puracé NNP, and the largest contribution to biomass consumed was also N. olivacea and Mazama sp. as in our results. Previous published studies from the high Andes are scarce, so the record of C. taczanowskii can be considered as the first record of this species in the puma’s diet across its distribution.
It is important to note that the consumption of Coendou rufescens by puma has been previously reported in the páramo of Belmira (2,800 to 3,000 masl, Antioquia department, north of the Central Andean range; Arias-Alzate pers. obs.); although this species is not found in Tamá NNP and it was not found on the scats collected; a similar species occurs in the area (i. e., Coendou pruinosus), so it could be expected in the diet. Chinchilla (1997) also highlighted a frequent consumption of Coendou mexicanus in the lowlands of Corcovado National Park, Costa Rica. Other potential prey species that were expected to be part of the puma’s diet in Tamá NNP, and that were previously recorded by camera traps, included Didelphis pernigra, Sciurus granatensis, Conepatus semistriatus, and Mustela frenata (Cáceres-Martínez et al. 2016). However, and despite they have been reported previously in the diet, we did not find them in the scats collected.
It seems that variations in puma’s diet are the result of opportunistic predation by this species, adapting its feeding behavior according to prey species availability both at the latitudinal and the altitudinal levels (Iriarte et al. 1990). Moreover, at high elevations or in regions sympatric with jaguars, pumas tend to prey more frequently on medium-sized species such as agoutis (Dasyprocta spp.), pacas (C. paca and C. taczanowskii), coatis (N. narica, N. olivacea and N. nasua), and deer (P. mephistophiles, Mazama gouazoubira and M. rufina) and apparently only prey on large mammals and other small species to supplement their diet (Nuñez et al. 2000, Novack et al. 2005, De Azevedo 2008, Hernández-Guzmán et al. 2011). However, the preferred prey size may also be limited by environmental conditions; for instance, puma typically prey more frequently on large species (frequently deer) in temperate zones (Dalrymple and Bass 1996, Cunningham et al. 1999, Moreno et al. 2006).
For some carnivore species, such as large felids (e. g., puma and jaguar), the lack of suitable habitats and prey species as a consequence of anthropic pressure (e. g., deforestation and hunting) may promote predation on livestock (Hoogesteijn and Hoogesteijn 2005, De Azevedo 2008). It seems that both habitat availability and the prey base at Tamá NNP are likely sufficient to avoid such behaviors, given that no livestock or other domestic species were found on the scats; pumas in Tamá may have no need to use such alternative prey. In other regions of Colombia (e. g., Antioquia), generally considered more impacted by humans, puma consumption of livestock has been frequently recorded (Arias-Alzate et al. 2013, González-Maya et al. 2013), indicating that conflict prevention, and the conservation of puma, depends on the maintenance of healthy ecosystems and local prey base via sustainable forest management and poaching control.
Our results represent significant knowledge of what is likely the most important Andean predator and they provide the baseline for understanding patterns of assemblage dynamics and thus for informing conservation. Changes in puma diet dynamics will likely reflect in changes in the composition and structure of mammal assemblages, not only represented as part of puma ecology, but also in general ecosystem dynamics and function. Our data can provide the base to understand the potential responses of species assemblages to changes in threats, both inside protected areas and surrounding areas, all increasingly dominated by human activities.
We highlight the importance of continuing long-term monitoring of these ecological processes, and in general with the generation of this type of studies in other regions of Colombia, to support better conservation strategies for species such as the puma, given the important roles it plays in ecosystem dynamics and stability in both protected and unprotected areas.











 nueva página del texto (beta)
nueva página del texto (beta)


