Introduction
Human activities such as agriculture and extensive cattle ranching, extraction of fossil fuels, overexploitation of resources and urbanization have all significantly impacted ecosystems worldwide (Hosonuma et al. 2012). Anthropic impact has affected the diversity and abundance of species, endangering the survival of their populations (Haddad et al. 2015; Ribeiro et al. 2015). In fact, organizations such as the International Union for the Conservation of Nature, as well as various authors, recognize that the accelerated loss of species and drastic ecosystem alterations have set off a global biodiversity crisis (IUCN 2017; Johnson et al. 2017). Conservation efforts and strategies to understand and/or reverse extinction trends generally focus on species that are either appealing or of interest to man (Donaldson et al. 2016). However, extinction processes associated with organisms perceived as harmful or associated with a negative condition, such as parasites, are poorly documented (Strona 2015).
Ecosystem disturbance (e. g., deforestation), defined by Rykiel (1985) as the set of processes that cause environmental disturbance (e. g., fragmentation and loss of habitat), disrupt ecological interactions at various levels, for instance, by changing food webs and mutualistic interactions (Morris 2010). The documented ecological changes between organisms involving several mammals species have shown that the host-parasite dynamics is one of the aspects affected in disturbed systems (e. g., marsupials, Puttker et al. 2008; rodents, Bordes et al. 2015). For example, it has been found that in colonization processes, transmission rates and the maintenance of parasitic infections decrease in hosts whose populations have been fragmented and isolated (Bush et al. 2013; Bordes et al. 2015). Therefore, a prevalent hypothesis in parasite ecology theory is that the diversity and abundance of parasites decrease as environmental disruption increases (Lafferty and Kuris 2005; Lafferty 2012).
The loss of parasite diversity may be aggravated in populations of hosts that are threatened to some extent (Farrell et al. 2015). Conwell et al. (2012) suggest that organisms such as parasites may undergo co-extinction along with their hosts, since the transmission of parasites is harder to sustain in isolated populations with low host density. In support of this hypothesis, Altizer et al. (2007) found in a meta-analysis that several species of threatened primates have a lower richness of parasite species versus unthreatened populations. The order Primates represents an excellent model to explore the effects of habitat disturbance on parasitism patterns in endangered hosts, since over 60 % of the species of primates are under some category of threat (Estrada et al. 2017), and most are habitat specialists. Therefore, assessing parasitic infection patterns in endangered primates provides valuable information for the conservation of hosts and their ecological interactions.
The black howler monkey (Alouatta pigra) is a Neotropical primate currently threatened of extinction due to habitat loss and the conversion with tropical forests into farming land (Estrada 2015; IUCN 2017). This species is endemic to Mesoamerica (Mexico, Guatemala and Belize) and 80 % of its distribution range is estimated to be located in southeastern Mexico in the states of Tabasco, Campeche, Quintana Roo, and northern Chiapas (Estrada 2015). The municipality of Balancán, Tabasco, is home to one of the populations of black howler monkey most severely affected by changes of land use (Pozo-Montuy et al. 2011). Between 1960 and 1975, the federal government promoted colonization programs coupled with extensive livestock raising and agriculture, which resulted in the loss of 115,000 hectares of forest and the conversion of both rainforests and the so-called tintales (vegetation dominated by Haematoxylum campechianum) into pastures and cropland (Isaac-Márquez et al. 2008). This municipality currently includes fragments of the original vegetation of different sizes not covered by government protection (Pozo-Montuy et al. 2008); for this reason, the populations of howler monkeys in this region have been proposed as a priority for preserving the species (Pozo-Montuy et al. 2008; Tobón et al. 2012). A parasitological investigation of this population may illustrate how habitat disturbance has affected ecological interactions between wild primates and other organisms.
The objective of this study was to document the effects of habitat disturbance on parasitism in black howler monkeys (A. pigra) inhabiting a landscape that has been highly impacted by human activities in the municipality of Balancán, Tabasco, Mexico. As habitat disruption affects the host-parasite interactions, we expected that increased levels of rainforest disturbance would reduce the presence and richness of GI parasites in the black howler monkey. Also, since parasitic infections may follow seasonal patterns and are affected by demographic and intrinsic aspects of hosts (2006), this study also considered seasonality as well as ecological density and sex of individual hosts as potential predictors of parasite presence and richness.
Materials and Methods
Study Area. The study was carried out in a fragmented landscape in the municipality of Balancán, Tabasco, Mexico (17° 40’ N, -91° 30’ W; Figure 1). The remnants of vegetation (i.e., fragments of howler monkey habitat, sensu Pozo-Montuy et al. 2013) in this area are located within a matrix of habitats that include livestock pasture, forestry plantations (cedar, Cedrela odorata; teak, Tectona grandis; melina, Gmelina arborea; and eucalyptus, Eucalyptus sp.), and cropland (e. g., sorghum, rice, maize). These fragments are scattered in an area of 21,900 hectares bordered by the Usumacinta river and various water bodies (Pozo-Montuy et al. 2008). The original vegetation in this area has been described as low and medium subdeciduous forest and tinto low thorny tropical forest (H. campechianum; López-Mendoza 1980; Pozo-Montuy et al. 2011). Annual precipitation is 1,976 mm and mean annual temperature is 27.9 °C (CNA 2017).
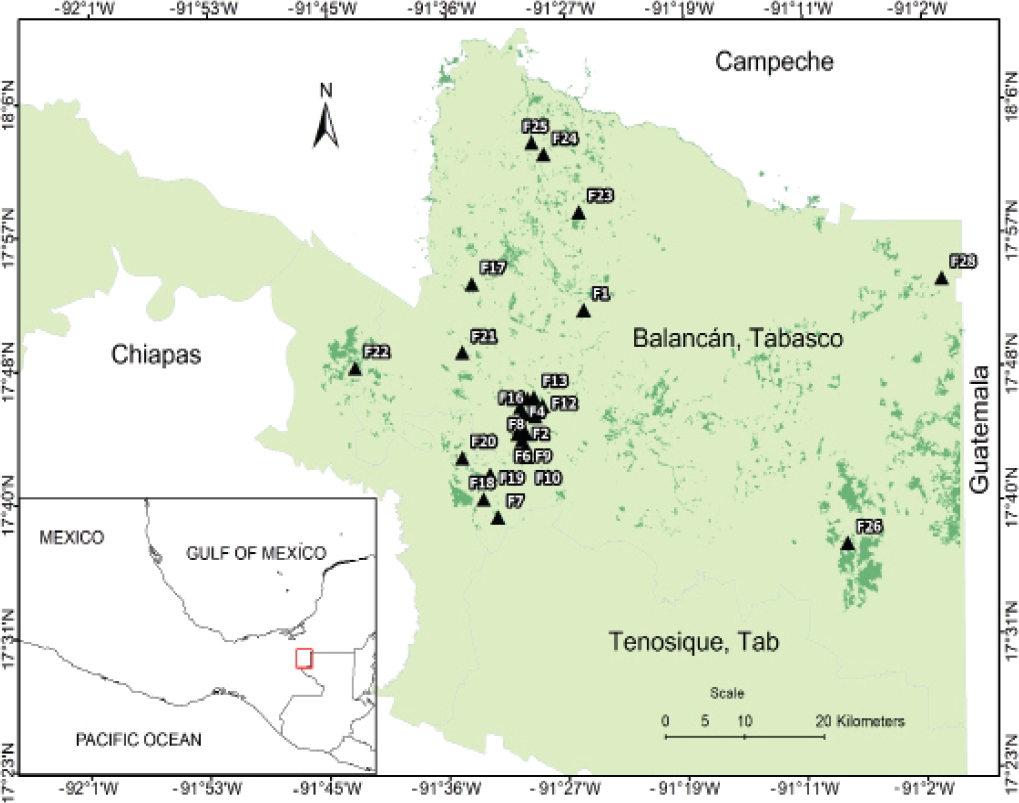
Figure 1 Fragmented landscape in the municipality of Balancán, Tabasco, Mexico. The image shows fragments of remnant forest in the study area, including the fragments sampled and human settlements.
Sample Collection. As part of a long-term research (2005 - 2018), complete censuses were conducted to record the presence of black howler monkeys, with 264 forest fragments visited during the period 2005-2012 (for further information, refer to Pozo-Montuy et al. 2008, 2011). Of these, and based on logistical criteria, a subsample of 30 fragments with confirmed presence of black howler monkeys was selected for the present study (Figure 1). Within the period January-December 2006, in each fragment the size and age-sex structure of howler monkeys was recorded, the ecological density (i. e., number of individuals per fragment size, Rodríguez-Toledo et al. 2003) was calculated, and fresh stool samples were collected from 33 males and 32 females of different groups (n = 65). Stool samples were collected opportunistically during population surveys and only when individuals could be identified. These samples were preserved in 10 % formaldehyde solution, labeled with the information of origin (e. g., fragment, group, specimen identity), and sent to the Laboratory of Parasitology in the Department of Environmental Sciences at Emory University.
Stool ova and parasite testing. We used the protocol of concentration by flotation and sedimentation (NaNO3 solution), as described in Gillespie (2006), to retrieve helminth eggs and cysts of intestinal protozoa excreted in howler monkey stools. This protocol is highly effective to estimate intestinal parasitic infections using non-invasive methods in various species of wild primates (Gillespie and Chapman 2008). Two slides were systematically examined under a light microscope (Leica® DM500) for each parasite concentration technique. This procedure was carried out for each stool sample. No intestinal protozoan cysts were found in these samples. Parasite eggs (i. e., helminths) were examined at 10× and measured with a micrometer eyepiece (0.1 μm) at 40×. One drop of Lugol’s iodine solution was added to highlight egg structures and facilitate the taxonomic identification. In addition, photographs of representative eggs were taken for use in the identification and classification of taxa (Figure 2). The recovery of eggs was used for estimating the presence, prevalence and richness of parasites as a measure of parasitic infection. Parasite richness was defined as the number of different taxa observed per stool sample, while parasite prevalence was defined as the proportion of stool samples infected with at least one taxon.
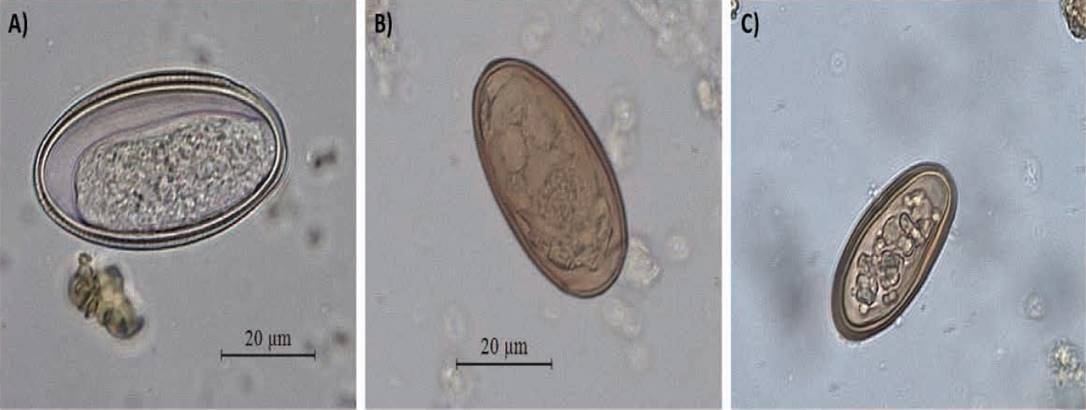
Figure 2 Parasite eggs found in stools of black howler monkeys that inhabit forest fragments in Balancán, Tabasco, Mexico. A) Trypanoxyuris sp., B) Controrchis sp., C) unidentified trematode.
Disturbance Measures. The size of each forest fragment occupied by monkeys and the distance from this fragment to the nearest village were considered as landscape measures of habitat disturbance. Both variables were estimated with orthophotos at scale 1:2,000 using the program ArcView 3.3. These data and estimates have been published in greater detail in Pozo-Montuy et al. (2008, 2011). Fragment size was included considering that larger vegetation patches can accommodate a larger number of individuals, species and ecological interactions, thus mimicking the conditions found in undisturbed habitats (Collins et al. 2017). The distance from each fragment to the closest village was included assuming that fragments in close proximity to human settlements are exposed to greater disturbance (e. g., logging and resource extraction, magnification of the edge effect; Popradit et al. 2015).
Data Analysis. The effects of habitat disturbance on parasitic infection patterns were analyzed using generalized linear models. The effects of habitat disturbance on the presence of parasites were explored by constructing a matrix with values 0 and 1 as response variables, where these figures represented non-infected (absence of parasites) and infected (presence of parasites of any taxon) samples, respectively. In this model, we established a canonical logit link function with binomial distribution (Crawley 2007). Fragment size and distance between a fragment and the nearest town were selected as predictors. Also included was ecological host density in each fragment due to a potential density-dependent effect on the parasitic infection (Nunn and Altizer 2006). Since samples were collected in different time points, these models also included seasonality. This was modeled through the sine and cosine of the day of collection, previously transformed into a circular variable (day within the year × 2 × π / 365) (Gillespie et al. 2013). The sex of specimens was also considered as a predictor.
In order to investigate the effects of habitat disturbance on parasite richness, we built another model including the number of parasite taxa in each stool sample as the response variable and the same predictors mentioned above. In this model, we established a canonical log link function and used the Poisson distribution (Crawley 2007). All models were run in the program R (version 3.2.4.) using the package MASS (Ripley et al. 2017). Taking into account all the predictors, an analysis for the selection of the best model was carried out using the “dredge” function of the package MuMIn (Barton 2017). This produces a series of potential combinations of the predictors previously specified. The best model for both the presence and richness of parasites was chosen according to the Akaike information criterion (Burnham et al. 2001). The significance of the best model to explain the data was evaluated with an analysis of deviance, which compares the goodness of fit of the best model vs. a null model that includes only the intercept. A plot of the significant effects of generalized linear models was drawn with the “effects” package (Fox et al. 2016) in R. Three stool samples belonging to one group were excluded from these analyses due to missing ecological information about the fragment. Parasitic prevalence values were calculated in the program Quantitative Parasitology 3.0 (Rózsa et al. 2000).
Results
This population of black howler monkeys showed a 39.1 % prevalence of intestinal parasites (95% CI: 27.1 to 52.1). On average, howler monkeys were infected by 0.5 ± SE 0.08 taxa (range: 0 to 3 taxa). The taxa found were Trypanoxyuris sp. (Oxyuridae), Controrchis sp. (Dicrocoeliidae), and an unidentified trematode of the family Dicrocoeliidae (Figure 2). Trypanoxyuris sp. eggs measured 47.9 µm ± SD 4.0 × 23.8 µm ± SD 1.4; Controrchis sp. eggs measured 43.1 µm ± SD 3.3 × 23.5 µm ± SD 1.4; and eggs of the unidentified trematode measured 37.7 µm ± SD 4.1 × 22.0 µm ± SD 1.0. Controrchis sp. was the most prevalent parasite (31.6 %), whereas the unidentified trematode and Trypanoxyuris sp. showed prevalence rates of 11.7 % and 5.0 %, respectively. Table 1 shows the prevalence of each taxon according to host sex and site of collection. The prevalence of intestinal parasites in black howler monkeys approximately doubled in individuals inhabiting fragments located more than 500 m away from the nearest village (Table 2). Considering fragment size, we found a similar prevalence of intestinal parasites in howler monkeys living in fragments varying from < 1 ha to 50 ha, but this increased by 43 % in larger fragments (>50-1200 ha) (Table 2).
Table 1 Prevalence of three taxa of gastrointestinal parasites associated with the sex of black howler monkeys (Alouatta pigra) inhabiting forest fragments in Balancán, Tabasco, Mexico.
| Trypanoxyuris sp. | Controrchis sp. | Unidentified trematode | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fragment | Size (ha) | Distance (m) | Male | Female | Male | Female | Male | Female | na |
| 1 | 0.01 | 250 | 0 | 0 | 0 | 20 | 0 | 0 | 5 |
| 2 | 0.01 | 2,500 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 3 | 0.2 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 4 | 0.8 | NA | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 5 | 1.4 | 500 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 6 | 1.5 | 950 | 0 | 0 | 100 | 0 | 0 | 0 | 1 |
| 7 | 1.9 | 600 | 0 | 0 | 50.0 | 0 | 0 | 0 | 2 |
| 8 | 2.1 | 870 | 0 | 0 | 0 | 100 | 0 | 0 | 1 |
| 9 | 2.5 | 1,580 | 0 | 0 | 0 | 100 | 0 | 100 | 1 |
| 10 | 2.8 | 120 | 0 | 33.3 | 0 | 33.3 | 0 | 0 | 3 |
| 11 | 3.8 | 1,019 | 0 | 0 | 100 | 0 | 0 | 0 | 1 |
| 12 | 4.1 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 13 | 4.5 | 670 | 0 | 0 | 0 | 0 | 100 | 0 | 1 |
| 14 | 4.7 | 1,214 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 15 | 4.9 | 2,400 | 0 | 0 | 100 | 0 | 0 | 0 | 2 |
| 16 | 6.7 | 400 | 0 | 0 | 20.0 | 0 | 0 | 0 | 5 |
| 17 | 11.2 | 2,500 | 50.0 | 0 | 50.0 | 0 | 0 | 0 | 2 |
| 18 | 14.5 | 708 | 0 | 0 | 0 | 50.0 | 0 | 0 | 2 |
| 19 | 17.0 | 780 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 20 | 21.9 | 2,408 | 0 | 0 | 0 | 0 | 0 | 50.0 | 2 |
| 21 | 24.4 | 100 | 0 | 0 | 14.2 | 28.5 | 14.2 | 14.2 | 7 |
| 22 | 25.0 | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 23 | 26.6 | 1,381 | 0 | 0 | 33.3 | 33.3 | 33.3 | 0 | 3 |
| 24 | 30.0 | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 25 | 39.0 | 560 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 26 | 76.9 | 250 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 27 | 166.0 | 1,235 | 0 | 0 | 0 | 0 | 0 | 100 | 1 |
| 28 | 200.0 | 1,200 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 29 | 288.5 | 950 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 30 | 1,200.0 | 455 | 0 | 0.25 | 0 | 50 | 0 | 0 | 4 |
a number of stool samples collected
NA: Information not available
Table 2 Prevalence of gastrointestinal parasites in black howler monkeys (Alouatta pigra) of Balancán, Tabasco, Mexico, in relation to distance between forest fragments and human settlements, and to fragment size.
| Predictive variables | Number of Fragments | Prevalence (%) | na |
|---|---|---|---|
| Distance between fragments and the nearest village | |||
| 100-250 m | 5 | 29.4 | 17 |
| >250-250 m | 6 | 26.7 | 14 |
| >500-1,000 m | 8 | 50.0 | 12 |
| > 1,000 m | 10 | 56.3 | 19 |
| Range of fragment sizes | |||
| 0.01-10 ha | 16 | 37.9 | 32 |
| >10-50 ha | 9 | 36.4 | 22 |
| >50-1200 ha | 5 | 55.6 | 11 |
a number of stool samples collected
The model found to best predict the presence of GI parasites considers the distance between fragments and closest village, as well as fragment size (Table 3). The analysis of deviance showed that the residual model including these two predictors significantly differed from the null model (χ2 = 6.4; d.f. = 2; P = 0.04). A generalized linear model indicated that the distance between forest fragments and the closest villages significantly predicted the presence of GI parasites (β = 0.55 ± SE 0.28, z = 2.0, P = 0.05). Figure 3 shows that the probability of intestinal parasitic infection increases with the distance between a fragment and the closest village. Although fragment size is an element of the best model, this predictor had no significant effect on the presence of parasites (β = 0.48 ± SE 0.30, z = 1.6, P = 0.11). Other predictors considered in this analysis had no significant effects on the presence of intestinal parasites.
Table 3 Results of the analysis for the selection of models that predict the presence of gastrointestinal parasites in black howler monkeys (Alouatta pigra) in Balancán, Tabasco, Mexico.
| Model | D. F. | Log-likelihood | AICc | ∆AICc | Weight |
|---|---|---|---|---|---|
| Distance + Size | 3 | -37.178 | 80.8 | 0.00 | 0.058 |
| Distance + Density + Sex | 4 | -36.206 | 81.1 | 0.36 | 0.048 |
| Distance + Size + Density | 4 | -36.354 | 81.4 | 0.65 | 0.042 |
| Distance | 2 | -38.679 | 81.6 | 0.78 | 0.039 |
| Distance + Density | 3 | -37.592 | 81.6 | 0.83 | 0.038 |
| Distance + Sex | 3 | -37.651 | 81.7 | 0.95 | 0.036 |
| Distance + Size + Sex | 4 | -36.662 | 82.1 | 1.27 | 0.031 |
| Distance + Size + Density + Sex | 5 | -35.547 | 82.2 | 1.42 | 0.028 |
| Distance + Density + Seasonality (sine) + Sex | 5 | -35.564 | 82.2 | 1.45 | 0.028 |
| Distance + Seasonality (cosine) | 3 | -37.971 | 82.4 | 1.59 | 0.026 |
| Distance + Density + Seasonality (sine) | 4 | -36.954 | 82.6 | 1.85 | 0.023 |
| Density + Seasonality (sine) | 3 | -38.103 | 82.6 | 1.85 | 0.023 |
| Distance + Size + Seasonality (sine) | 4 | -36.956 | 82.6 | 1.86 | 0.023 |
| Size | 2 | -39.219 | 82.6 | 1.87 | 0.023 |
| Distance + Seasonality (sine) | 3 | -38.139 | 82.7 | 1.92 | 0.022 |
| Seasonality (sine) | 2 | -39.289 | 82.8 | 2.00 | 0.021 |
Models are listed from lowest to highest value of the Akaike Information criterion (AICc)
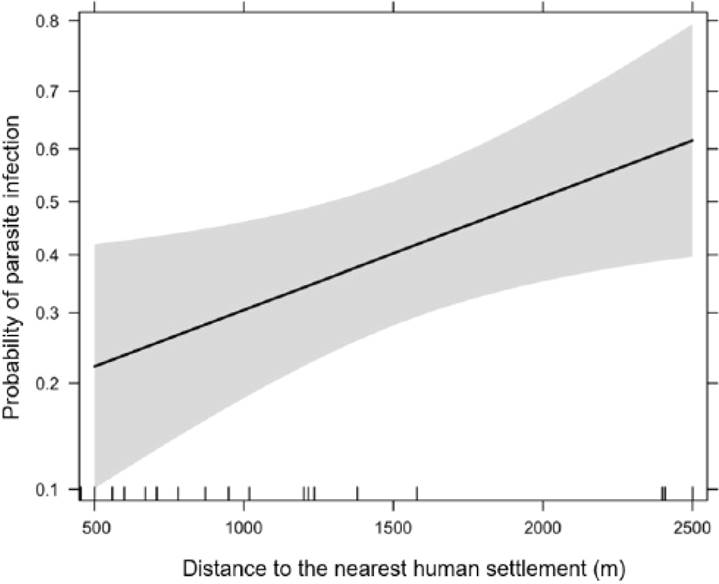
Figure 3 Relationship between distance (m) from forest fragments to the nearest village and likelihood of intestinal parasitic infection in black howler monkeys living in Balancán, Tabasco, Mexico. The shaded area represents 95 % confidence intervals.
Ecological density was the best predictor of parasite richness in the model selection analysis; however, this model had no significant effects (analysis of deviance: χ2 = 2.4; d. f. = 1; P = 0.12). The other predictors considered also showed no significant effects on parasite richness.
Discussion
In the present investigation, we conducted an ova and parasite stool study to assess the effects tropical forest disturbance on parasitic infection patterns of in black howler monkeys threatened with extinction. We found that as the extent of disturbance increases — estimated through the proximity of human settlements to forest fragments — the presence of parasites in this population of primates decreases. Also, a higher prevalence of parasites was observed in monkeys inhabiting fragments further away from villages. These findings suggest that infection rates, and probably also the transmission processes of parasites infecting black howler monkeys, are disrupted as anthropogenic activities are intensified in the proximity of fragments.
In this population of primates, we also found that parasite richness (n = 3 taxa) is low relative to the one reported for howler monkey populations living in undisturbed environments (Table 4). In these populations, individual monkeys carry a higher number of parasite species, ranging from 4 to 9 taxa. In contrast to our results, a study carried out in forest fragments in the southern region of the municipality of Balancán, Tabasco, reported 13 taxa of parasites, 11 of which showed a prevalence of 2 to 10 % (Alvarado-Villalobos 2015). Despite the higher number of taxa found at that site, the low prevalence indicates that infections involving multiple taxa are rare in the area. It is possible that the variability observed in the recovery of parasite species partly resulted from the different parasitological techniques used in other investigations (Alvarado-Villalobos et al. 2017); however, our results are consistent with the richness reported for other monkey populations inhabiting fragmented areas (Table 4).
Table 4 Richness and prevalence of gastrointestinal parasites in different populations of black howler monkeys (Alouatta pigra)
| Habitat | Sitea | Number of individuals | Number of stool samples | Richness | Range of prevalence | Taxab | Reference |
|---|---|---|---|---|---|---|---|
| Undisturbed | LAR, CBS | 167 | 167 | 6 | 2.0 - 80.8 | 1, 12, 20, 30, 31, 32 | Eckert et al. 2006 |
| CBS, CBWS, PA, CK | 50 | 283 | 4 | 27.0 – 81.0 | 6, 12, 18, 28 | Vitazkova and Wade 2006 | |
| MA | 15 | 151 | 8 | 25.0 – 35.0 | 3, 12, 13, 17, 21,24, 25, 33 | Stoner and González Di Pierro 2006 | |
| CK, ES, PA, MA | 137 | 137 | 4 | 2.5 – 17.0 | 6, 23, 27, 37 | Trejo-Macías et al. 2007 | |
| PT, TG | 8 | 8 | 9 | 12.5 – 50.0 | 2, 7, 8, 9, 11, 13, 15, 16, 18 | Bonilla Moheno 2002 | |
| Fragmented | MR | 17 | 221 | 5 | 11.1 - 88.9 | 1, 5, 26, 27, 36 | Behie et al. 2014 |
| MC | 22 | 22 | 3 | 7.7 – 50.0 | 23, 24, 35 | Trejo-Macías et al. 2007 | |
| PC | 43 | 218 | 3 | 9.1 – 73.0 | 6, 9, 27 | Alvarado-Villalobos 2010 | |
| ES | 15 | 258 | 5 | 0.7 – 49.0 | 6, 13, 22, 27, 34 | Martínez-Mota et al. 2017 | |
| PL, PH | 3 | 3 | 6 | 33.3 - 100 | 2, 7, 11, 13, 16, 18, | Bonilla Moheno 2002 | |
| SB | 41 | 492 | 13 | 2.4 – 83.0 | 1, 3, 4, 5, 9, 10, 12, 14, 17, 19, 24, 27, 30 | Alvarado-Villalobos 2015 | |
| T, C | 10 | 46 | 4 | 2.2 - 23.9 | 24, 27, 28, 29 | Solórzano-García and Pérez-Ponce de León 2017 | |
| MB | 65 | 65 | 3 | 5.0 - 31.6 | 5, 27, 34 | This study |
a LAR: Lamanai Archaeological Reserve, Belize; CBS: Community Baboon Sancturary, Belice; CBWS: Cockscomb Basin Wildlife Sanctuary, Belice; PA: Palenque, Chiapas; CK: Calakmul, Campeche; MA: Montes Azules, Chiapas; ES: Escárcega, Campeche; PT: Petcacab, Quintana Roo; TG: Tres Garantías, Quintana Roo; MR: Monkey River, Belice; MC: Marqués de Comillas, Chiapas; PC: Playas de Catazajá, Chiapas; PL: Punta Laguna, Quintana Roo; PH: Pacchen, Quintana Roo; SB: Sur de Balancán, Tabasco; MB: Municipio Balancán, Tabasco; T: Tabasco; C: Chiapas.
b 1 Ascaris sp.; 2 Balantidium coli; 3 Blastocystis sp.; 4 Chilomastix sp.; 5 Controrchis sp.; 6 C. biliophilus; 7 Cryptosporidium sp.; 8 Cyclospora sp.; 9 Eimeria sp.; 10 Endolimax sp.; 11 E. nana; 12 Entamoeba sp.; 13 E. coli; 14 E. histolytica; 15 E. hartmanni; 16 E. poleki; 17 Enterobius sp.; 18 Giardia sp.; 19 Hymenolepis nana; 20 Iodamoeba butschlii; 21 Isospora sp.; 22 Parabronema sp.; 23 Raillietina sp.; 24 Strongyloides sp.; 25 Trichostrongyloides sp.; 26 Trichuris sp.; 27 Trypanoxyuris sp.; 28 T. minutus; 29 T. pigrae; 30 Reported as oxyurid; 31 Nematode larvae; 32 Unidentified trematode; 33 Unidentified trematode; 34 Unidentified trematode; 35 Unidentified trematode; 36 Reported as Trichostrongylidae; 37 Unidentified parasite.
The low probability of occurrence of parasites in the proximity to human settlements and the low richness recorded may be associated to the high degree of ecosystem disturbance and changes of land use in the municipality of Balancán, Tabasco. In particular, this area is recurrently affected by fires associated to pasture burning and practices such slash-and-burn in cropland areas surrounding forest fragments (Manjarrez Muñoz et al. 2007). These activities alter the microclimate of the forest floor, considerably reducing soil moisture and increasing desiccation (Laurance 2004). These changes have been reported to reduce the survival of infectious stages of parasites (Bloemers et al. 1997). The low probability of occurrence of parasites in this population of howler monkeys likely reflects the synergy resulting from the reduction in the parasite-host encounter rates (Bordes et al. 2015) and the low resilience of parasites to survive in a highly disturbed ecosystem.
The parasites reported in this research are considered to be specialists, as they have been found specifically in a monophyletic group of Neotropical arboreal primates (family Atelidae; Solórzano-Garcia and Pérez-Ponce de León 2017). It has been proposed that host-specialized parasites tend to be more susceptible to extinction compared with generalist parasites, which manage to persist in the environment by parasitizing a greater variety of hosts belonging to different evolutionary lineages (Farrell et al. 2015). In this context, the decline of howler monkey populations may also lead to the loss of parasites that infect only this endemic primate species. For instance, Solórzano-García et al. (2016) have recently described the species Trypanoxyuris pigrae, a nematode that parasitizes specifically A. pigra and that may be threatened of extinction along with its host.
On the other hand, the high discontinuity of the canopy in some forest fragments in this area forces howler monkeys to descend to the ground to move across treeslocated either within fragments or in the matrix of fragmented landscapes (Pozo-Montuy and Serio-Silva 2007). It has been proposed that this shift in behavior increases the likelihood of contact between primates and infectious stages of parasites deposited in soil and whose primary hosts are humans or livestock (Rwego et al. 2008; Zommers et al. 2013). However, in this study we found none of the parasite species reported in humans or domestic animals. In a recent research, Helenbrook et al. (2015) explored the possibility of cross-transmission of the parasite Blastocystis sp. between mantled howler monkeys (A. palliata aequatorialis) and humans coexisting in close proximity, but found no evidence of transmission between species. Pedersen and Davies (2009) suggest that the transmission of parasites between host species is more likely to occur in phylogenetically closer taxa. As the evolutionary relationship between monkeys and either humans or domestic animals is distant (Perelman et al. 2011), this could explain the absence of parasites shared between monkeys and humans/livestock despite the frequent contact of this population of primates with human settlements and farming activities in the area. Further stool ova and parasite studies using molecular techniques may provide compelling information concerning the parasite transmission processes between black howler monkeys and other species.
Our results contrast with those obtained in other studies that have investigated the parasite ecology of wild primates. For example, in colobus (Procolobus rufomitratus) and macaque (Macaca silenus) monkeys, it has been found that individuals who live in highly disturbed forests show higher rates of GI parasitism (Gillespie and Chapman 2008; Hussain et al. 2013). These shifts in susceptibility to parasitic infections are seemingly related to nutritional stress (Chapman et al. 2015). However, recent studies on black howler monkeys show that these primates are able to prevent nutritional stress by consuming food items of different nutritional quality and energy content, supplementing the diet with food gathered across the landscape matrix (Pozo-Montuy et al. 2013; Martinez-Mota et al. 2016). An alternative hypothesis is that the intake of secondary metabolites from plants through the diet might reduce parasitic infections (Forbey et al. 2009). Tinto (H. campechianum) is a plant frequently consumed by howler monkeys in the study area, which contains secondary compounds such as phenols, flavonoids and gallotannins (Kandil et al. 1999). These compounds may negatively affect the viability of parasites, as previously reported in experimental studies (Athanasiadou and Kyriazakis 2004). This hypothesis deserves further investigation. The trend observed in our study indicates that changes in host-parasite dynamics in relation to habitat disturbance are specific for each biological system (Salkeld et al. 2013).
Despite the fact that parasitic infections are often associated with a negative or poor health condition in animals, parasites are members of healthy ecosystems, functioning as a link in food webs, and making a significant contribution to global biodiversity (Marcogliese 2005; Shea et al. 2012; Sukhdeo 2012). Our study suggests that habitat disruption affects not only populations of appealing species, but also the persistence of parasite communities, which is reflected on the patterns of gastrointestinal parasite infections in wild primates that depend strictly on tropical forests.











 text new page (beta)
text new page (beta)


