Introduction
The bobcat (Lynx rufus) is one of the carnivores with the broadest distribution in North America, stretching from southern Canada to the Baja California peninsula and Oaxaca in Mexico (Hall 1981; Romero 1993; Larivière and Walton 1997; Romero 1993). This carnivore inhabits a range of environments, from pine, pine-oak and tropical deciduous forests, scrubs and grasslands, to areas with high human density, coexisting with domestic animals and wildlife (Gehrt et al. 2010). The bobcat has shown tolerance to fragmented environments (Riley et al. 2004), hence increasing the likelihood of contact with dogs and cats, with which it may potentially share the parasitic load (Harrison 1998; Medellín and Bárcenas 2009; Prough et al. 2009; Carver et al. 2012; Koleff et al. 2012; Valencia-Herverth and Valencia-Herverth 2012; Hiestand et al. 2014).
Parasites reported for the bobcat are generalists of wild and domestic mammals of the Order Carnivora, including Alaria marcianae, Taenia rileyi, Physaloptera Cylicospirura praeputialis, Felineus, Toxascaris leonina, T. mystax and Ancylostoma tubaeforme for West Texas (Stone and Pence 1978), while Paragonimus kellicotti, Spirometra mansonoides, T. macrocystis, T. leonina, T. mystax, A. tubaeforme, Oslerus rostratus, Molineus barbatus, Physaloptera rara and Troglostrongylus wilsoni were reported as typical components of the bobcat parasitic community for West Virginia and Georgia (Watson 1981). In a recent study, T. leonina, A. caninum, Ancylostoma spp., and Taenia spp. were reported as parasite species shared with other wild carnivores, such as coyotes (Canis latrans) and pumas (Puma concolor) (Hiestand et al. 2014), while L. rufus shares Toxocara cati with dogs and cats, mainly when the former lives in urbanized areas where contact with domestic animals has been reported (Riley et al. 2004; Keesing et al. 2006; Carver et al. 2012; Mino-Botello et al. 2016).
In recent years, a number of ecological studies have been conducted on the bobcat in Mexico, making it possible to broaden its distribution range with new records (Valencia-Herverth and Valencia-Herverth 2012), identifying differences in feeding patterns (Medellín and Bárcenas 2009), or evaluating the effect of landscape attributes on its presence (Botello et al. 2006; Monroy and Briones-Salas 2012; García- Prieto et al. 2014; López-González et al. 2015; Espinosa-Flores and Lopez-González 2016), to mention a few examples. In Mexico, only three parasite species have been formally registered for the bobcat, according to the information available in the National Collection of Helminths at the Institute of Biology, UNAM. These records correspond to two nematodes, Toxascaris leonina and Physaloptera praeputialis, and one cestode, Echinococcus oligarthra (Salinas-Lopez et al. 1996; García-Prieto et al. 2012). Specifically, for the study area, there is one study on the presence of a cyst in the intestinal mucosa of a bobcat, and the presence of the nematode P. praeputialis is reported for the first time for the state of Querétaro (Lopez-González et al. 2012). Last, the presence of endoparasites has been reported in several mesocarnivores inhabiting the Ajusco, including L. rufus (Gallardo 2014).
The scarce biological information available on parasites of wildlife hinders the development of prevention and control strategies and measures in the event of potential zoo-noses of importance for humans. Toxocara cati is considered as a species with zoonotic potential. Previous studies have reported a higher prevalence of this species in areas where wild and domestic animals are found in sympatry, in turn increasing the likelihood of contact with humans (Bevins et al. 2012).
The objective of this study was to determine the composition of the parasite community in the faeces of the bobcat (Lynx rufus) inhabiting areas surrounding the city of Queretaro, and testing for significant differences in parasite composition and prevalence between seasons. Based on the available information on the composition and structure of the parasite communities of the bobcat living in disturbed environments in the United States. We expected to find a community of intestinal parasites dominated by generalist species, typically associated with the presence of domestic animals and habitat loss, in areas surrounding the city of Queretaro.
Materials and Methods
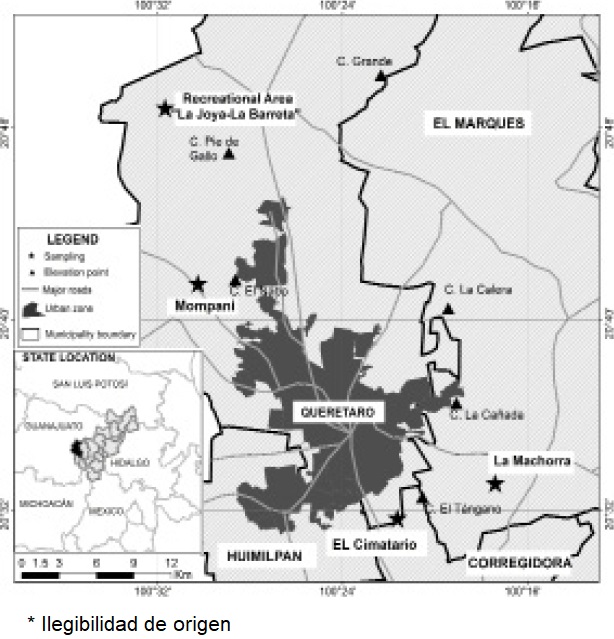
Study Area. The study was carried out in four collection sites in areas surrounding the city of Queretaro: El Cimatario National Park (PANEC), Joya-La Barreta Recreational Area, ExHacienda La Machorra and the area adjacent to Los Angeles dam in Mompani, all sites where the presence of Lynx rufus has been reported (Figure 1).
El Cimatario National Park is surrounded by peripheral suburbs of the city of Queretaro. It is located at 20.474° to 20.556° N, and -100.326° to -100.386° W, with a reported area of 2,447 ha. It is a protected natural area dominated by crasicaule (thick-stemmed) scrub and tropical deciduous forest (Baltasar et al. 2004; López-González et al. 2015). The PANEC is listed as a protected natural area where grazing, reforestation, firewood collection, hiking and cycling activities take place.
The Joya-La Barreta Recreational Area, located in the Santa Rosa Jauregui delegation, is part of the Santa Catarina micro-watershed in the boundary between the States of Queretaro and Guanajuato (20.809° N, - 100.528° W). It comprises six vegetation types, mainly oak forest, natural grassland, petrophytic vegetation (in ravines and gullies) and crasicaule scrub. Its primary functions are ecological conservation and special protection, but subsistence activities such as grazing and soil and firewood extraction are conducted in the area, as well as recreational activities like camping and hiking. The area of influence of the park includes locations such as La Barreta, La Carbonera and La Joya, where primary-production activities such as maize cultivation, nomadic livestock raising and firewood collection for domestic use are performed (Hernández-Sandoval et al. 2005).
Mompani is a rural town where the “Los Angeles” dam is located, in coordinates 20.711° N, -100.506° W. It is surrounded by secondary vegetation, crasicaule scrubs, agricultural areas and hills covered with tropical deciduous forest where livestock raising and firewood collection for domestic use are conducted (Pineda-Lopez et al. 2010).
Ex-Hacienda “La Machorra” is located in the municipality El Marqués between communities El Rosario and Los Cués, in coordinates 20.554° N, -100.286° W. The land is covered by crasicaule scrub vegetation where livestock raising is not practiced but materials such as gravel and stone are extracted. To note, groups of feral dogs were observed in and around collection sites during sampling periods, due to the proximity of human settlements.
Scat Collection and Examination. Tracks were surveyed weekly in search of scats and latrines during the rainy season (late May, July, September and October 2015) and the dry season (early March and May 2016). Surveys were performed at 1-week intervals to ensure the independence of individual scats collected, as suggested in Hernández-Camacho et al. (2011).
Scat samples were identified in the field based on shape (cylindrical and in clusters), size (approx. 1.5 to 2.5 cm wide by 10.0 to 15.0 cm long), color (usually light gray or blackish when fresh), composition (mostly hair and bone remains), and association with lynx tracks adjacent to scats (Aranda 2012). Only fresh scats that did not break apart upon handling were sampled, to avoid weathering and ensure the potential presence of parasite dispersal stages. Scat samples were split into two halves, one was placed inside a polyethylene bag with zipper and fixed with 10 % formalin following the methodology of Hernández-Camacho et al. (2011). The other half was placed in a paper bag to be tested for coproculture and as source of replicate samples. Scat samples were labeled with the respective collection data (sample number, date, collection site and georeference), and were stored at 4 °C until analysis.
Stool ova, parasite and coproculture tests. Stool samples were analyzed with the Ritchie float or formalin-ether test to extract protozoan eggs, cysts and oocysts (Medina et al. 1994; Hernández-Camacho et al. 2011; Aranda et al. 2013). Parasite eggs were identified based on morphometry under a LEICA ICC50 HD light microscope using the software LAS EZ Leica Application Suite version 3.1.1. Scat cultures and larval migration tests were conducted aiming to obtain L3 larvae (Medina et al. 1994). The taxonomic identification of eggs was confirmed under the microscope, by comparison with the specialized literature (Taylor et al. 2007; Bowman et al. 2011).
Statistical analysis. The total prevalence of parasites by locality was expressed in percentage and was quantified as the number of scat samples infected with any parasite divided by the total number of scats per collection locality and multiplied by 100. A Chi-square (X2) test was performed to assess potential differences between the collection seasons. The species composition of parasite communities was compared between sites and between seasons through a non-metric multidimensional scaling (NMDS) analysis, which is usually the method of choice for the graphical representation of relationships at the community level (Clarke 1993). This analysis was performed using the Jaccard similarity index and the software PAST 2.17c (Hammer et al. 2001).
Results
A total of 83 scat samples were collected for both seasons, 53 and 30 during the rainy and dry seasons, respectively. Parasite eggs were identified to the lowest taxonomic level possible, identifying a total of seven nematode species for the bobcat: Trichuris vulpis, P. praeputialis, T. leonina, T. cati, S. stercolaris, Uncinaria stenocephala and Ancylostoma sp.
A total prevalence of 75.9 % (n = 63) was observed in the two collection seasons. For the rainy season, 73.6 % of scat samples (n = 39) tested positive for at least one of the six nematode species. S. stercolaris was the species with the highest prevalence in the four sites (33.3 % in La Machorra and 100 % in El Cimatario); the species with the lowest prevalence were T. leonina (13.3%) and T. vulpis (20 %), which were found in only one site (La Machorra). For the dry season, 80 % of scats tested positive (n = 24), with T. cati and S. stercolaris as the most prevalent parasites, both with a 100 % prevalence in El Cimatario; T. vulpis showed a very low prevalence, observed only in Mompani (12.5 %). The X2 test showed no significant differences in nematode species between seasons (X2 = 0.553, d. f. = 1, P = 0.457). The non-metric multidimensional scaling (NMDS) analysis revealed no differences in species composition between seasons (Figure 2). Hence, this parameter was similar across collection sites. T. leonina was found only during the rainy season, and U. stenocephala, during the dry season; both parasite species were found only in La Machorra.
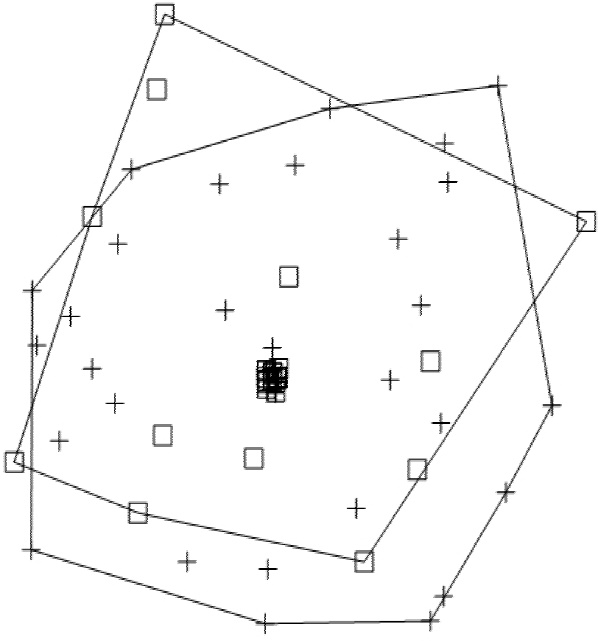
Figure 2 Plot of the non-metric multidimensional scaling of the species composition between the two seasons. White boxes indicate parasites recorded during the rainy season; crosses, parasites recorded in the dry season. Polygons represent seasons, the overlap shows that the composition of parasite communities is similar across sites, regardless of season.
Discussion
The parasitic load of the bobcat in areas surrounding the city of Querétaro consists of nematodes; this differs from what is recorded for scat samples collected in the United States, where a greater parasite diversity has been observed, including trematodes, cestodes, protozoans, and acanthocephalans (Stone and Pence 1978; Watson 1981; Tiekotter 1985; Larivière and Walton 1997; Hiestand et al. 2014); however, all the nematode species recorded are generalists of carnivores, which is consistent with our expectations. The technique used for the analysis of stools is suitable for the detection of the dispersal stages of many parasites (Hernández-Camacho et al. 2011). The presence of nematodes as the only parasite group may be the result of the different dispersal and transmission strategies used by these organisms, favoring their survival in anthropic environments, as is the case of the host-infection strategy or larva migrans, which penetrates through the skin of foot pads, as well as transplacental and transmammary transmissions (Anderson 2000). This great variety of options contrasts with those in other parasite groups, such as trematodes, cestodes and acanthocephalans, all of which require trophic transmission via intermediate hosts to complete their life cycle in the definitive host (Bush et al. 2001).
Some of the nematode species found in this study have also been recorded in feral dogs from the outskirts of Querétaro, such as S. stercoralis and Ancylostoma sp. (Fernández et al. 2002; Cantó et al. 2011), and in the gray fox (Urocyon cinereoargenteus), also with S. stercoralis (Hernández-Camacho et al. 2011), to mention a few examples. T. vulpis is recorded for the first time in L. rufus for Mexico at La Machorra and Mompani; this parasite was previously observed in scat of gray fox at El Cimatario (Hernández-Camacho et al. 2011).
Strongyloides stercoralis showed a 100 % prevalence in the dry season at El Cimatario National Park. This finding may indicate the contact of bobcats with humans and domestic animals, as suggested in the study of Mino-Botello et al. (2016) in the Tehuacán-Cuicatlán Biosphere Reserve. In the specific case of El Cimatario, the presence of packs of feral dogs has been documented (Hernández-Camacho et al. 2016), which interact with wild carnivores (Hernández-Camacho pers. com. 2017). This is similar to the situation mentioned by Mino-Botello et al. (2016).
The identification of Ancylostoma sp. to species level was not possible, despite the observation of distinctive characters in eggs and larvae obtained from scat cultures. However, A. tubaeforme and A. caninum have been recorded in bobcats in the United States (Stone and Pence 1978; Watson 1981; Hiestand et al. 2014). In the area adjacent to Santiago de Queretaro, A. caninum has been observed in stools of feral dogs and gray foxes; therefore, the Ancylostoma eggs and larvae found in this study likely correspond to this species.
Toxocara cati is the most prevalent parasite in wild and domestic cats (Bowman 2011). In this study, its prevalence was low relative to the other nematodes found. This parasite can be transmitted directly when eggs are ingested or through paratenic hosts such as mice of the genus Peromyscus (Aranda et al. 2002; Bowman et al. 2002; García-Prieto et al. 2012).
No significant differences were found in the composition of the parasite community between seasons, except for T. leonina and U. stenocephala in La Machorra. The presence of T. leonina in the rainy season may be due to its life cycle being favored by moisture and low temperatures, as well as by the presence of eggs and L3 larvae in tissues of rodents of the genus Peromyscus (Anderson 2000; Bowman et al. 2011). The species recorded during the dry season may be fostered by the presence of artificial water bodies in the study sites, which are favorable for the survival of the dispersal stages. Other studies have reported that the eggs of Toxocara sp. and Trichuris sp. are resistant to various environmental factors, and hence remain viable in soil for months (Mizgajska 2001; Rendón-Franco et al. 2013), until there are suitable conditions for completing their life cycle.
Each of the four localities in this study show some degree of anthropization, from El Cimatario National Park, an area under conservation strategies, to areas with intense human activity in La Machorra, associated with the extraction of construction materials, and Mompani, related to the Santiago de Queretaro landfill. All the localities studied recorded parasite species that are generalists and resilient to environmental disruption, which may point to the historical presence of human activities in these sites (Hernández-Camacho et al. 2016).
The parasite species found are generalists typical of carnivores, with life strategies that allow them to survive and persist in environments with anthropic activities. This could indicate that the areas surveyed in this study have undergone a steady long-term loss of biodiversity, where the intermediary hosts of several parasite species are no longer in the area (Lafferty 1992; Lafferty 2012, Hernández-Camacho et al. 2016). On the other hand, domestic and wild carnivores interact as predators and competitors. This leads to a significant impact on wild populations, with a negative effect on the trophic structure of the remaining ecosystems in the areas around cities. This may have negative health effects on local inhabitants, by being increasingly closer to zoonotic parasites (Riley et al. 2004; Gehrt et al. 2010; Sepúlveda et al. 2014).











 nova página do texto(beta)
nova página do texto(beta)