Introduction
Females of the white-tailed deer Odocoileus virginianus (Zimmermann 1780) display complex behavioral patterns during pregnancy, birth and breeding, probably aimed at maximizing offspring survival (Kie and White 1985; Wallace 1990; Wallace and Krausman 1990; Schwede et al. 1993). Breeding fawns is likely to modify the type of areas used by females, as well as foraging and anti-predator strategies, and social responses (Gilliam and Fraser 1987; 1989; Holzenbein and Schwede 1989; Schwede et al. 1994; Main et al. 1996; Bowyer et al. 1998; Bongi et al. 2008; DeYoung and Miller 2011). It is generally accepted that parturition induces a temporary reduction in home-range size, habitat use, and social interactions (Nelson and Mech 1981; Ozoga et al. 1982; Holzenbein and Schwede 1989; Nixon et al. 1992; Zultowski 1992; Schwede et al. 1993; Fox and Krausman 1994). The only published study comparing female deer with and without fawns found no differences in home-range size (Bertrand et al. 1996).
In northeastern Mexico, a number of studies have compared the behavior of males and females of the subspecies O. v. texanus (Gallina et al. 2003), by analyzing the use of habitat between years, sexes and reproductive periods (Bello et al. 2001a, 2003), home range (Bello et al. 2001b), movements in relation to precipitation (Bello et al. 2004), distances traveled (Bello et al. 2006), energy expenditure (Gallina and Bello 2010), and activity (Gallina and Bello 2014). The current knowledge about pregnancy and fawning is limited. It is considered that home-range size and habitat use directly influence the deer population dynamics (Gallina 1981; Gallina et al. 1998; Green et al. 2017), reproductive patterns (gestation, number of offspring, time of parturition, fawn survival, size of mothers and fawns), and the behavior of mothers (Green et al. 2017). The objective of this study was to determine the behavioral strategies of females of white-tailed deer with and without fawns under conditions of semi-captivity, estimating home-range size and use of plant associations in a brushland.
Materials and Methods
Study Area. This work was carried out at Rancho San Francisco, located between the municipalities of Lampazos and Progreso in the States of Nuevo León and Coahuila (27° 22’ N, -100° 40’ W; 27° 22’ N, -100° 36’ W; 27° 20’ N, -100° 40’ W; 27° 20’ N, -100° 36’ W). The study area stretches across 1,500 ha, including a 1,000 ha enclosed in a deer fence, with 33 artificial 1500-L water troughs in addition to three dams whose water level depends entirely on rainfall (Bello et al. 2001a). Maximum temperature can reach 40 °C, and the maximum mean temperature in July reaches only 29 °C (Briones 1984). The climate is warm and dry, with mean annual precipitation below 400 mm; the rainy season spans from May to September, showing interannual variations (Bello et al. 2001a; Bello et al. 2004). The estimated deer density is 8 to 10 individuals per km2, and between 80 and 100 deers are estimated to thrive in the area (Gallina and Bello 2010; Bello et al. 2001b).
The local vegetation is a xeric scrubland, including seven plant associations with variable cover: 1% medium-height thorny mezquite-acacia brushland, 3% toboso grassland (Hilaria mutica = Pleuraphis mutica), 6% thornless hojasen shrubland (Flourensia cernua), 10% chaparro prieto (Acacia-Celtis), 11% cenizo shrubland (Leucophyllum frutescen), 15% high thorny mezquite-acacia shrubland (Prosopis), and 54% medium sub-thorny mezquite-acacia-toboso-hojasen shrubland (Acacia-Prosopis; Bello et al. 2001b). Factors considered were availability of each association, species richness, cover protection and percent of food species in each association.
Although the predation of adult females and fawns was not documented in this study, the presence of puma (Puma concolor), bobcat (Lynx rufus), feral dog (Canis lupus familiaris) and coyote (Canis latrans) was recorded in the study area, all being potential predators (Cook et al. 1971; Ozoga and Verme 1982; Messier and Barrette 1985). Breeding coyote couples predate on deer fawns, mostly during July and August (Lopez-Soto and Badii 2000).
Capture and Radio Tracking. Females were captured using a 15 x 15 m drop net with a 20 cm mesh modified for capturing deer, with a manual trigger that is activated from a distance of approximately 30 m. Corn grains were used as bait. When a deer was captured, eyes were covered (to keep it calm); then it was untangled from the net and the legs were tied to facilitate handling. This procedure is commonly used in the UMAS located in the States of Nuevo León, Coahuila and Tamaulipas. Each deer was weighed with a Pesola 100 kg dynamometer and was fitted with a 180 g radio transmitter collar with activity sensor (Model 400, Telonics, Inc. Meza, Arizona). No anesthetic were administered to avoid complications in deer handling (Bello et al. 2001a).
Eight females were captured, six in 1997 and two in 1998 (Table 1). Three were captured within the 1,000 ha confinement area, and five outside of this area. The six females monitored in 1997 were raising fawns. The six females monitored in 1998 had no fawns.
Table 1 Year of capture and number of monthly locations of females of Texan white-tailed deer in Rancho San Francisco, Nuevo León.
| Female | Year of capture |
Number of locations with fawns |
Number of locations without fawns |
||||||||
| Apr | Jun | Aug | Oct | Total | May | Jun | Aug | Oct | Total | ||
| 1 | 1997 | 14 | 29 | 45 | 31 | 119 | 46 | 30 | 41 | 28 | 145 |
| 2 | 1997 | 10 | 20 | 12 | 33 | 75 | 36 | 21 | 33 | 25 | 126 |
| 3 | 1997 | 16 | 35 | 38 | 30 | 119 | 47 | 41 | 43 | 30 | 161 |
| 4 | 1997 | - | 30 | 52 | 30 | 112 | 30 | - | 25 | 25 | 80 |
| 5 | 1997 | 42 | 19 | 20 | 19 | 100 | - | - | - | - | - |
| 6 | 1997 | - | - | 56 | - | 56 | - | - | - | - | - |
| 7 | 1998 | - | - | - | - | - | 33 | 15 | 14 | 34 | 96 |
| 8 | 1998 | - | - | - | - | - | 18 | - | 17 | 13 | 48 |
| Total | 82 | 133 | 223 | 143 | 581 | 240 | 107 | 209 | 180 | 736 | |
All the females studied were located using radio telemetry (White and Garrot, 1990). Female locations were recorded in May, July, August and October 1997, and in April, July, August and October 1998, corresponding to the breeding season (Table 1). Data were obtained at 1-h intervals, performing two to three 24 hour cycles each month. Each deer specimen was radio-located by triangulation using TR2 and TR4 receptors (Telonics Inc., Meza, Arizona;) and H- and Yaguis-type portable antennas (Telonics Inc., Meza, Arizona) from two or three fixed stations (Samuel and Fuller 1994) fitted with a Garmin georeferencing instrument, performing simultaneous readings. We used a Suunto KB-14 (Finland) compass to obtain the directions for each triangulation.
Estimated Home Range. To obtain the location of each female deer, degrees were transformed to UTM coordiates for each triangulation using the software Tripoly 2 (Laundré 1990), considering a magnetic deviation of 9.15° and an error of 0.7 ha. The home-range size of each individual deer female was estimated with the CALHOME Program (California Home Range; Kie et al. 1994) using a 95% minimum convex polygon model (White and Garrot, 1990). A total of 1,317 locations were recorded (581 in 1997 and 736 in 1998). The number of locations used to calculate the home range was similar for each individual female in the two years (Table 1).
To perform the analysis, the breeding season was divided into four stages regardless of the female reproductive status: 1) pre-breeding stage (April to June), corresponding to the late gestation period. 2) First breeding stage (July), when fawns are completely dependent on the mother. 3) Second breeding stage (August), when fawns start becoming independent. 4) Third breeding stage (October), corresponding to the total weaning of fawns. The results were analyzed at two levels: a) variations in each breeding stage considering the home range of the eight females captured, and b) variations in each stage at the individual level, considering only three of the four females monitored in 1997 (with fawns) and 1998 (without fawns). The fourth female was excluded from the analysis because no records of its activity were noted during April (1997) and June (1998).
The data were not normally distributed, nor the variances were homogeneous, so that non-parametric analyzes were used (Zar 1996). The variations in home-range size was explored for each stage at the sample level (n = 8) between females with and without fawns using Kruskal-Wallis and Mann-Whitney tests (Zar 1996). The monthly home-range size at the individual level for the three females monitored during the two years was analyzed using Friedman tests. The home-range size of females with and without fawns was compared using Wilcoxon tests (Zar 1996).
Use of and Preference for Plant Associations. Only the three females that were observed with and without fawns were considered. An analysis was made throughout the entire breeding season and another for each breeding stage, considering the availability of each plant association in both Rancho San Francisco and the home range of each female. We used the ArcView geographic information system software to obtain a digitized map of the home range of each female, derived from the locations recorded through radio telemetry for each plant association. The habitat use and preference were derived for each female from the number of locations in each plant association. The use of associations according to availability was assessed with X2 tests (Zar 1996) and Bonferroni intervals (Byers et al. 1984).
Results
Home Range. Home-range size varied between females with and without fawn across the stages analyzed (H = 16.84, P = 0.01). In females with fawns, it was largest in the pre-breeding and second-breeding stages (U = 2, P = 0.05; U = 1.0, P = 0.01, respectively). The home-range size between stages was similar in females with fawns (H = 4, P = 0.20). Females without fawns (H = 9, P = 0.02) showed a smaller home range from the pre-breeding stage to the first and second breeding stages (Figure 1).

Figure 1 Home range of females with and without fawns of Texan white-tailed deer during the breeding season.
At the individual level, the three females monitored in 1997 versus 1998 showed a similar home-range size across stages. However, there was a trend to display larger home-range sizes when raising fawns and in the pre-breeding stage, that did not reach statistical significance (n = 3; Xr2 = 12, P > 0.05). A similar size across stages was observed in both females with fawns (Xr2 = 5, P > 0.05) and females without fawns (Xr2 = 7, P > 0.05). These females relocated their home range in each stage, showing a different overlap between each breeding stage (Figure 2).
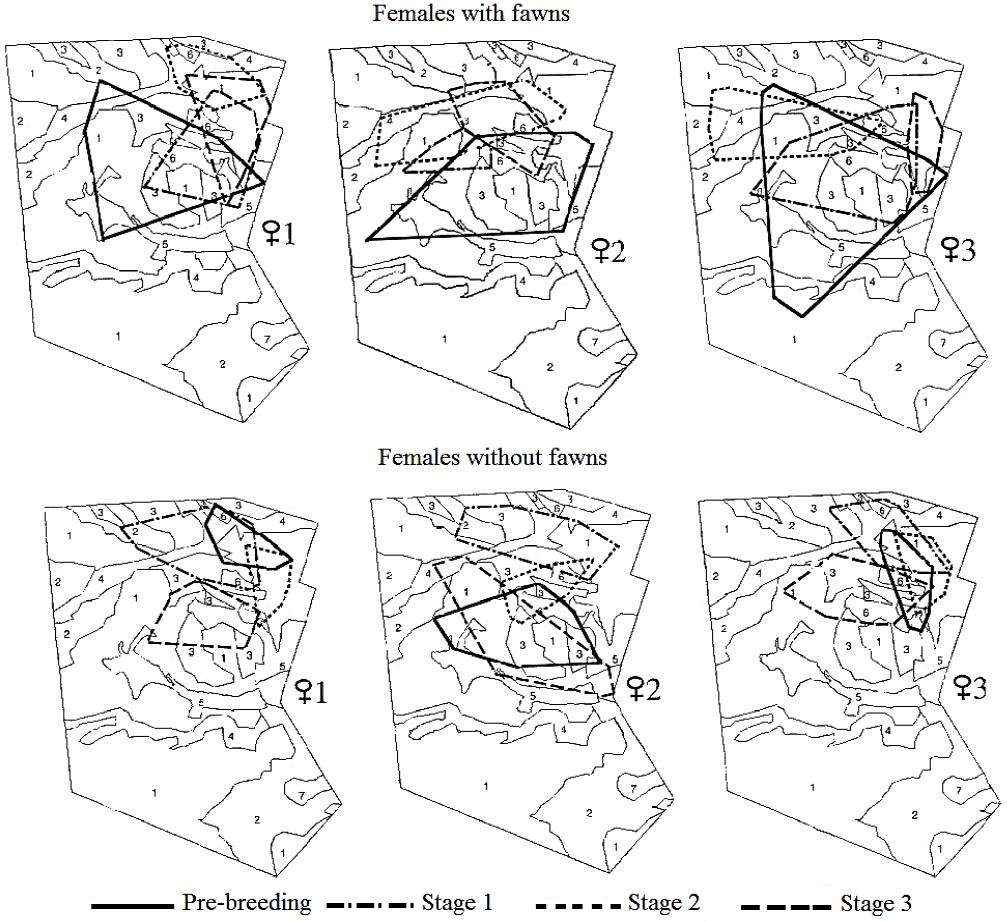
Figure 2 Location of the home range of females of Texan white-tailed deer with and without fawns during the breeding season. Plant associations: Acacia-Prosopis (1); Prosopis (2); Leucophyllum (3); Acacia-Celtis (4); Flourensia (5); Hilaria (6); Opuntia (7).
Use of and Preference for Plant Associations. Irrespective of their reproductive status, females included six of the seven plant associations in Rancho San Francisco within their areas of activity: Acacia-Prosopis, Leucophyllum frutescens, Prosopis, Acacia-Celtis, Flourensia cernua and Hilaria mutica (Figure 2). The number of observations corresponding to each plant association differed for each of the females, within an interval that ranged from 0 locations in Opuntia up to 91 locations in Acacia-Prosopis (Figure 3).

Figure 3 Use of and preference for plant associations by females in each breeding stage: w/f with fawns and w/of without fawns. Symbols: greater-than expected use (+); lower-than-expected use (-); expected use (unsigned); association not included in the home range (Ni).
During each breeding stage, females displayed a differential use of the plant associations. females with fawns (female 1: X2 = 16, d. f. = 8, P = 0.001; female 2: X2 = 33, d. f. = 8, P = 0.001; female 3: X2 = 41, d. f. = 10, P = 0.001) and without fawns (female 1: X2 = 30, d. f. = 10, P = 0.001; female 2: X2 = 37, d. f. = 10, P = 0.001; female 3: X2 = 37, d. f. = 10, P = 0.001). The proportion of home-range use differed between stages and females showed a preference for Acacia-Prosopis only (Figure 3). No difference was found in the behavior of females with and without fawns in the three breeding stages (Females 1, 2 and 3; P < 0.03).
Discussion
Females with fawns showed larger home ranges in the pre-breeding and the second breeding stages, which is consistent with the report by Bertrand and collaborators (1996). This may be due to the increased energy metabolism in the last gestation stage (30 to 45%) rather than in lactation (Gallina and Bello 2010). In Rancho San Francisco during the breeding season, females increase their energy expenditure to 100 Kcal/ind/day (Gallina and Bello 2010), as well as the time dedicated to food search and feeding to consume herbaceous and shrub sprouts (Gallina et al. 1998). By contrast, the lack of fawns may foster optimal resource use within smaller areas, as females restrict their activity sites (Shipley and Spalinger 1995). Females with and without fawns relocated their home range in each breeding stage, which is common in deer, according to resource distribution and abundance, as well as social factors (Mackie 1970; Riley and Dood 1984; Tierson et al. 1985).
Females with fawns do not show a significant change in home-range size between pre-breeding and the three breeding stages, as reported in other studies (Zultowsky 1992; Beltrand et al. 1996). As reproduction was successful, all females seemingly obtained the resources needed simply by relocating their home ranges. The area used is likely the minimum area that allows them to obtain the resources needed to meet their energy demands and those of fawns in each breeding stage.
The most important plant associations for females during the breeding season are Acacia-Prosopis and Leucophyllum frutescens. These associations show an intermediate cover (39.5% and 34% respectively), and a near-to-highest species richness (10 sp), surpassed only by Flourensia cernua (11 sp). Acacia-Prosopis showed an intermediate proportion of species consumed by deer (64.7%), while Leucophyllum frutescens had the lowest percentage of such species ( 28.4%; Bello et al. 2003). Therefore, habitat selection by females is seemingly determined by an intermediate cover value coupled with a high diversity of plant species.
Females use areas of intermediate plant cover during the breeding season, maybe as an anti-predator strategy. This cover allows fawns to hide in the vegetation while still facilitating a quick escape (Nelson and Mech 1981; Kie and White 1985; Schwede et al. 1994; Bowyer et al. 1998; Mandujano et al. 2004; DeYoung and Miller 2011); in addition, fawns obtain protection from high sun radiation and rain-fall (Messier and Barrett 1985; Illius and Gordon 1987; Fox and Krausman 1994; Bowyer et al. 1998).
Nursing females apparently carried out an optimal foraging strategy, as observed in other sites (Marchinton and Hirth 1984; Hofmann 1989). They choose plant associations with high species diversity (Arceo et al. 2004), seeking food quality rather than abundance (Weckerly and Kennedy 1992; Weckerly 1994; Main et al. 1996; Kammermeyer and Larry 1997; Gallina et al. 1998), and inhabit areas with low food biomass if these increase the safety of their offspring against predation (Miquelle et al. 1992).











 nueva página del texto (beta)
nueva página del texto (beta)


