INTRODUCTION
The Chocó biogeographic region, stretching from southern Panama to northern Ecuador, is one of the regions of greater richness and biological uniqueness worldwide (Hernandez-Camacho et al. 1992; Morrone 2001), covering the western (Pacific) slope of the Andes, from sea level to 2,000 m (sensu Gentry 1982; Olson and Dinerstein 2002), This biological uniqueness is due to the presence of ecosystems as varied as the Chocó trans-Andean rainforests, the Chocó-Darien humid forests, the montane humid forests of Panama and the Chocó-Esmeraldas mangroves (Holdridge et al. 1971; Hershkovitz 1982; Hernández-Camacho et al, 1992; Arias-Alzate et al. 2012), These ecosystems, communicated with the inter-Andean valleys of the northern end of the Western and Central Cordilleras, are dispersal routes for lineages originated in South, North and Central America (Hernández-Camacho et al, 1992; Morrone 2001), Therefore, the Chocó is a region of great importance for understanding the systematics and historical biogeography of many vertebrate species (Morrone 2001; Arias-Alzate et al, 2012),
A total of 84 rodent species are currently recorded for the Chocó biogeographic region (Méndez 1993; Tirira 2010; Solari et al. 2013), many of which display a continuous distribution throughout the region, Groups like the genus Orthogeomys, although showing high diversity from southern Mexico to Central America, have a low species richness in the north tip of South America (Goldman 1920; Hall and Kelson 1959; Méndez 1993), In this region, Serrania del Darién, Serrania de los Saltos and Serrania del Baudó form a mountain range that apparently function as a geographical barrier restraining the dispersal of the terrestrial fauna toward southern areas (Alberico 1990; Sudman and Hafner 1992; Valdés-Velásquez 2003; Hafner 2015), However, some authors argue that in the case of fossorial species this mountain range does not represent a definitive biogeo-graphical barrier (Alberico 1990; Monge 2010),
Historically, the presence of O. dariensis in the region had been reported in several locations in the eastern end of Panama, near Serrania del Darién between 80 and 750 m on the border with Colombia (Goldman 1920; Handley 1966), For its part, O. thaeleri was described by Alberico (1990) and is known from several localities across central Chocó between 30 and 100 m, Until now, these populations had been treated as different species based on differences in pelage and cranial features (Alberico 1990), However, due to the low genetic divergence between individuals assigned to both species, 0,3 % for the cytochrome-b mitochondrial gene (Sudman and Hafner 1992) and 0 % for two nuclear genes (Spradling et al. 2016), O. dariensis and O. thaeleri are currently considered as a single species (Hafner 2015; Spradling et al, 2016),
Given the few distributional and ecological studies on these populations (Goldman 1920; Alberico 1990; Correa and Perea 2007), their geographical ranges remain little known, Moreover, the spatial and temporal variability of their niches, which would allow an approximation to the evolutionary history of the species, remain are unknown, In this investigation, stemming from the hypothesis that O. dariensis s.s. and O. thaeleri are two allopatric populations, we evaluated the degree of ecological segregation between these populations through an approximation of ecological niche models and niche conservatism hypotheses.
METHODOLOGY
Species records. Records of the presence of O. dariensis (northern and southern populations) were gathered through a revision of the specimens deposited in biological collections of Universidad Tecnológica de Chocó (Quibdó) and Instituto Alexander von Humboldt (Villa de Leyva); in addition, the relevant information in the scientific literature was reviewed (e. g., Alberico 1990; Reid 2009; Hafner 2015). For each record, the data gathered included locality and geographical coordinates (in decimal degrees), height above sea level and source of the records. The characteristics of soil were described according to the Soil Geographic Databases compendium (www.soilgrids.org).
Climate Information. Given the scarce knowledge on the taxonomy, ecology and distribution of these taxa (Goldman 1920; Alberico 1990; Correa and Perea 2007), and to avoid subjectivities in the selection of the most relevant climate variables that determine the distribution of O. dariensis, 19 bioclimatic variables were used from the general circulation model CGCM31 developed by CCMA (Canadian Center for Climate Modeling and Analysis), downloaded from the WorldClim webpage (http://www.worldclim.org, Hijmans et al. 2005). All variables were processed with a resolution of 30 arc-seconds (equivalent to 1 km2 or 0.008333 degrees).
Ecological niche models and potential distribution. Numerous methods for the study of ecological niches and geographic distributions are currently available, which have been compared extensively (Elith and Graham 2009; Elith et al. 2011; Peterson et al. 2011). For this reason, since our objective focuses mainly on ecological and evolutionary aspects of isolated populations rather than on the comparison of these methods, this study was conducted using the MaxEnt algorithm, a heuristic method that has produced satisfactory results (Elith et al. 2011; Santika 2011). The ecological niche modeling with MaxEnt estimates the probability of occurrence of a species through space, by comparing the ecological conditions in which the species has been recorded vs. a sample of background pixels of the study area using a Bayesian adjustment procedure and under the maximum-entropy principle (Phillips et al. 2006; Phillips and Dudik 2008). The predictions derived represent hypotheses on conditions similar to those where the species has been observed; the result is often interpreted as the potential distribution of the species (sensu Peterson et al. 2011).
From the hypothesis that O. dariensis has two populations (corresponding to O. dariensis s. s. and O. thaeleri as defined by Alberico 1990), two strategies were developed to model the potential distribution ranges. In the first strategy, the geographic distributions are estimated and compared considering two separate populations; in the second strategy, the species is modeled as a single taxon (O. darien-sis s.l.). These distributions were derived by dividing the total number of records (G+, sensu Peterson et al. 2011) in two data sets: a calibration data set (75 % of the data) and an evaluation data set (25 %), the procedure was repeated 100 times (100 replicates, each with 500 iterations; and each data set with different random partitions) using the bootstrap method (Phillips et al. 2006; Phillips and Dudik 2008).
As little is known about the ecological features and dispersal ability of this species, two areas (M; sensu Peterson et al. 2011) of different size (small and large) were produced to explore whether each individual population considered separately predicted the distribution of the other. Given the geographical characteristics of the region between Panama and Colombia, areas of 4,560 km2 and 25,500 km2 were generated for the northern population; and areas of 5,700 km2 and 24,800 km2, for the southern population. In addition, an analysis of multivariate environmental similarity surfaces (MESS; Elith et al. 2011; Saupe et al. 2012) explored the extent of the climatic differences between these areas, and whether some variables show values outside of the calibration area (the area where the model was produced) that might be influencing predictions (area where the model was projected) to a significant extent. The fitness, performance and discriminating capacity of the models were evaluated through the ROC (Receiver Operating Characteristic) curve and the AUC (Area Under the ROC curve) value, based on validation data (Phillips et al. 2006; Muscarella et al. 2014). The AUCTEST has proved to be a useful tool for ordinal scoring models (McPherson 2004; Thuiller et al. 2005; Santika 2011; Muscarella et al. 2014). The key variables for each population were identified using the Jacknife test (incorporated in MaxEnt).
Subsequently, a climatic characterization of the distributions in terms of temperature and precipitation was carried out for the analysis of the potential climatic segregation between populations, which would effectively validate a bias in terms of the geographical representativeness of populations. These variables have shown a good environmental resolution to interpret the interactions between species and their environment (abiotic factors) (see Tocchio et al. 2015; Qiao et al. 2016). Likewise, this characterization was performed for the Darién-Baudó, the geographic area in between the two separate distributions (see results), which apparently would function as a geographical barrier restraining the dispersal of these and other populations to southern areas in the region (Alberico 1990; Sudman and Hafner 1992; Valdés-Velásquez 2003; Hafner 2015). All subsequent processing and analyses were carried out in a geographic information system using the software ArcGIS 10.1 (ESRI 2014).
Similarity and conservatism of ecological niches. After exploring and estimating the distribution range for each population separately and for the two populations combined, we conducted an ecological niche similarity test as a proxy for niche conservatism (Wiens and Graham 2005). To this end, both the individual ellipsoids of each separate population and the one of the overall species were estimated in the ecological space using probabilistic models (MaxEnt output) as the hypothesis of the existing fundamental niche (EA) (Soberon and Nakamura 2009; Peterson et al. 2011; Qiao et al. 2016). This was conducted by means of the similarity and ecological niche overlap algorithm using the program Niche Analyst (NicheA; Qiao et al. 2016). Given the difficulty to estimate and visualize the multi-dimensional ecological space and the subsequent representation of niches in such space, a principal components analysis (PCA) was performed with climatic variables to reduce the dimensionality and hence visualize the ecological space in three dimensions (three principal components, X, Y, Z), in order to depict the ellipsoids. This overlapping analysis was used to calculate the volume of the ellipsoids corresponding to the three ecological niches, with their respective cen-troids, eigenvectors and percentage of shared volume. The similarity between ecological niches was measured with the Jaccard index, which produces values between 0 and 1, indicating the degree of similarity between niches in the ecological space (Qiao et al. 2016).
RESULTS
Ecological niche models and potential distribution. A total of 15 records of the species were recorded, six for the northern population (O. dariensis s.s.) and nine for the southern population (O. thaeleri), following the nomenclature proposed by Alberico (1990; Table 1). Both estimates of the distributions showed good performance and discriminating capacity (AUCTEST = 0.995 ± 0.005). The variables identified as most important for the two groups were precipitation in the coldest trimester, temperature seasonality, and temperature in the coldest month.
Considering both populations separately, geographical distributions are allopatric and associated with very humid forests with high precipitation and low temperature sea-sonality, being separated by the Darién-Baudó zone, which appears as an area that delimits the two distributions (Figure 1). The approximate distribution of the northern population includes the far south of Panama, mainly in the Province of Darién, which shows heavily leached red and yellow soils, with accumulation of clay, low cation-exchange capacity and low base saturation, stretching up to the northern portion of Serranía del Baudó and the alluvial valleys of Atrato and San Juan rivers, in Colombia. The distribution of the southern population is restricted to lowlands and soft non-alluvial soils in south-central Serranía del Baudó and the southern portion of the alluvial valleys of Atrato and San Juan rivers, from 0 up to 100 m, in the Pacific region of Colombia (Figure 1). For its part, when considered as a single species, it shows a continuous distribution range across an area of approximately 117,233 km2, and the Darién-Baudó zone is no longer displayed as a splitting area (Figure 1). However, although favorable conditions for the potential distribution of O. dariensis occur to the Pacific coastal plains, its southern distribution limit is possibly the San Juan river, which becomes a barrier for the species (Figure 1).
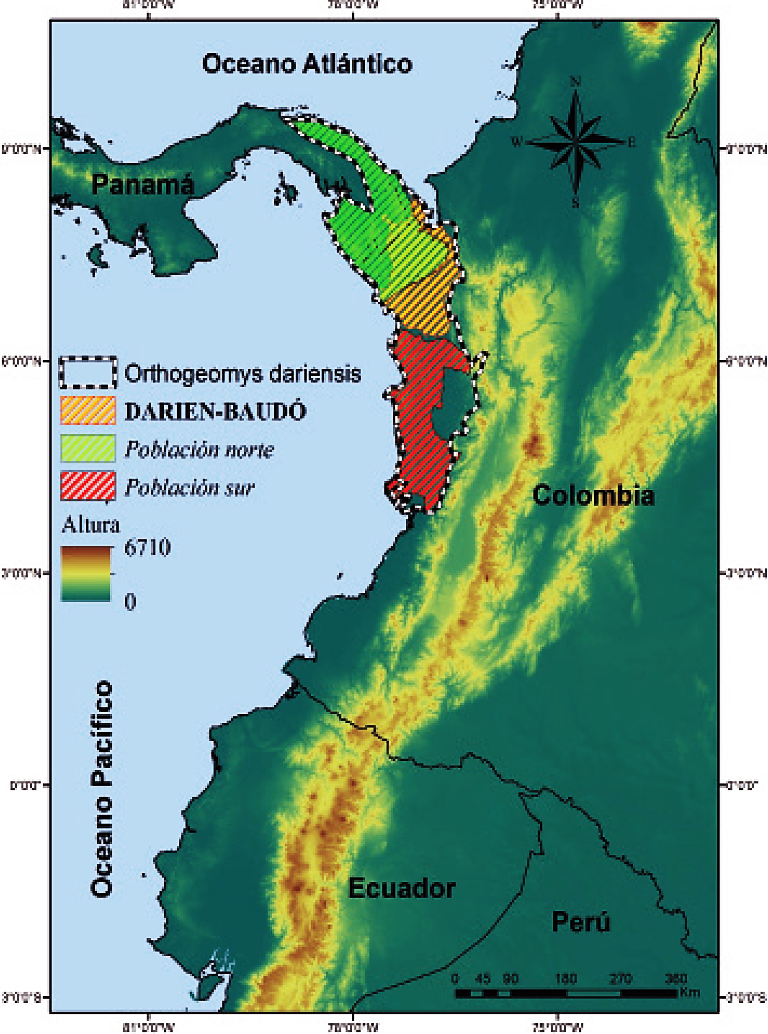
Figure 1 Study area and potential distributions of the northern population (green), southern population (red), and O. dariensis (dotted line) in the Chocó biogeographic region.
Considering the areas evaluated, both the small and the large area (for each species), the northern population predicts to a large extent the distribution of the southern population, and vice versa (Figure 2). On the other hand, the MESS analysis and the environmental variables associated with each area show that, although these areas share similar climatic conditions, there are some particular environmental characteristics (P < 0.05), which apparently do not represent conditions so unique as to separate these populations to any significant extent (Figure 3).
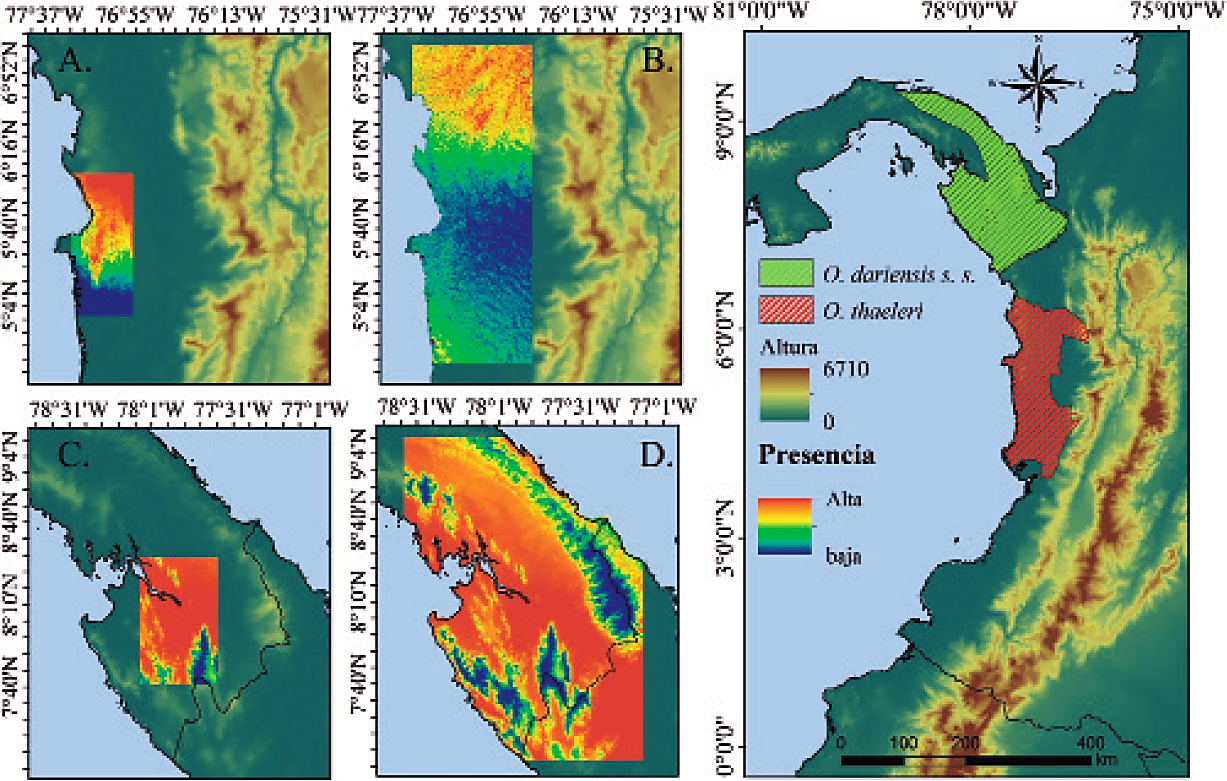
Figure 2 Potential distribution and predictions of the two populations of Orthogeomys dariensis in the Chocó biogeographic region. Prediction of the presence (A. small area; B. large area) of the northern population based on the southern population. Prediction of the presence (C. small area; D. large area) of the southern population based on the northern population. Note the Darien-Baudo zone as a potential barrier when two allopatric populations are considered.
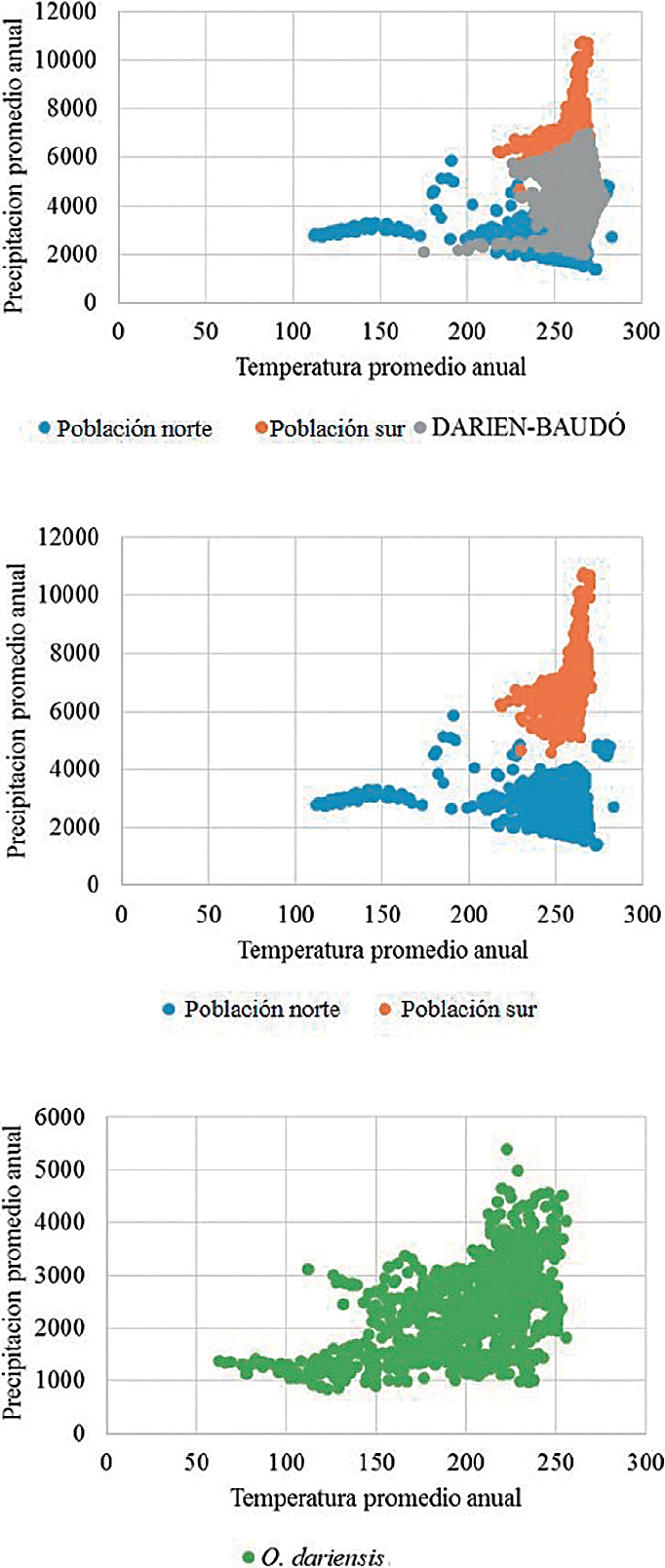
Figure 3 Climate analysis of the distributions of the northern population (blue), southern population (orange), the Darién-Baudo intermediate zone (gray), and O. dariensis as a single species (green) in the Chocó biogeographic region.
Similarity and conservatism of the ecological niche. According to the niche similarity analysis in the ecological space, both populations show a slight overlap of their ellipsoids (Jaccard index = 0.30; Figure 4). However, these hyper-volumes are subdivisions of a much larger niche when a single species with a continuous distribution area is considered (Figure 4). In this way, more than niche conservatism or divergence between the two populations, these findings reflect the ecological and environmental conditions associated with each population, one to the north and the other in a location closer to the center of the Chocó biogeographic region.
DISCUSSION
Among the various groups of small non-flying mammals that colonized South America during the Great American Biotic Exchange after the establishment of the Panamanian Bridge (Marshall et al. 1982; Cody et al. 2010; Almendra and Rogers 2012), apparently the least successful, as evidenced by their limited dispersal in South America (Simpson 1950), are shrews (Woodman and Pefaur 2008) and pocket mice of the genus Heteromys (Anderson 2000). However, for fosso-rial species belonging to the family Geomyidae, this dispersal was seemingly even more restricted, having inhabited and undergone diversification mainly in Central America.
In this region, O. dariensis (sensu Hafner 2015) shows two allopatric populations, with a southern range reaching the San Juan river (4.20° North latitude). These is consistent with the genetic analyses by Spradling et al. (2016), which show a very low genetic divergence in mitochondrial and nuclear DNA between these populations (Spradling et al. 2016). This is also supported by the morphometric analyses of Hafner (2015) and Spradling et al. (2016), who suggest that the morphological variability between the Colombian and Panamanian populations is insufficient to consider them as different species.
Likewise, the slight overlap of the ecological niches of the two populations reflects the particular ecological conditions in the areas they inhabit. Rather than considering the Darién-Baudó zone as a barrier restraining the dispersal and promoting the diversification for these gophers (Alberico 1990; Sudman and Hafner 1992; Valdés-Velásquez 2003), it may represent an area that connects both populations, Furthermore, Correa and Perea (2007) and Alberico (1990) point out that the burrow systems of populations in the Colombian Chocó are built in "inorganic clay" soils, with low-to-medium plasticity, and with scarcely developed profiles due to the continuing input of sediments from the Torreidó river; these characteristics are similar to those found in the Darién-Baudó intermediate zone (mainly in Medio Baudó, Chocó), which would facilitate the mobility and dispersal of populations in the region.
In order to gain a more comprehensive understanding of the evolutionary history and distribution of this geomyid species in the Chocó region, further studies are needed to confirm the presence and gather additional records, mainly for the Darién-Baudó zone, where no records are currently available. However, we believe that the species might be present based on its physical and environmental characteristics (see above). Such studies would contribute to a better understanding of the role of the Darién-Baudó (with an extension of 50 km wide approximately) as a dispersal route for populations of South and Central America. These responses are key to understand geographical and ecological differentiation patterns, not only regarding O. dariensis, but also with respect to the biotic and abiotic processes that have taken place in the region.











 nueva página del texto (beta)
nueva página del texto (beta)




