Introduction
Natural and man-made disturbance often result in discontinuous landscapes and shifts in land cover (Cuarón 2000) representing habitat loss and habitat fragmentation for many wildlife species. These are among the primary drivers of population declines and species extinctions (Caughley et al. 1996). Once a habitat gets fragmented, its resource availability shifts, producing variations in animal population densities and distributions (Pulliam and Danielson 1991). This situation may be analyzed using the source-sink system approach, where source fragments provide most of the essential resources for the survival and reproductive success of wildlife species. Therefore, natality rates usually are higher than mortality rates in source habitat, which may get to the point of reaching carrying capacity in such fragments. Surplus individuals may migrate into lower quality (sink) fragments, where mortality overcomes natality. Consequently, local populations in sink habitats rarely persist in the long term without immigration from source fragments (Pulliam and Danielson 1991; Hanski and Simberloff 1997).
A population may be distributed across source and sink fragments, giving place to subpopulations with different growth, natality, and mortality rates depending on available resources (e.g. food and shelter; Pulliam and Danielson 1991). When the resources within a habitat are used with higher frequency in relation to their availability, selectivity is inferred (Johnson 1980). The use of resources and their importance for a particular species vary as function of a series of factors that should be recognized to better evaluate the habitat requirements of animal species (Chalfoun and Martin 2007), especially those affected by habitat loss and unregulated hunting, as it happens to the spotted lowland paca Cuniculus paca (Cuarón 2000; Urquiza-Haas et al. 2009). The paca is a solitary and nocturnal Neotropical rodent. Pacas rest during the day inside underground cavities where their newborns are raised and kept apart from both predators and adverse weather conditions. This mammal occurs in a wide array of ecosystems such as different kinds of tropical forests, montane forests, mangroves, and riverine vegetation (Pérez 1992). Pacas feed mainly on fruit, contributing to seed dispersal and seed predation of many tropical tree species (Pérez 1992; Dubost and Henry 2006). Pacas are also important food items for predators such as jaguars (Panthera onca), pumas (Puma concolor), and ocelots (Leoparduspardalis; Pérez 1992; Eisenberg and Redford 2000). Besides, the paca has always been a primary prey for Neotropical subsistence hunters, who regard this species as very relevant in cultural and nutritional terms (Weitlaner 1997; Corona and Enriquez 2011). Currently, this mammal is heavily pursued by hunters in every place it can be found, particularly in southern Mexico, where it is often the most hunted and consumed wild mammal due to the relative easiness of its capture and the excellent taste of its meat (Naranjo et al. 2004; Centeno and Arriaga 2010).
The paca is relatively tolerant to habitat fragmentation and land use change in the tropical forests of Mexico (Cuarón 2000; Gallina et al. 2012). During the second half of the twentieth century, over 50% of the original cover of the Lacandon Rainforest, Chiapas, Mexico has been lost (Vásquez-Sánchez and Ramos 1992). Habitat loss and fragmentation coupled to heavy hunting pressure in southern Mexico (Naranjo et al. 2004), may be primary drivers to paca population declines and even local extinctions (Estrada et al. 1994). Thus, it is of utmost importance to improve our knowledge on paca populations and their habitat requirements in the wild as well as in transformed landscapes. This information will support sound management plans and actions for sustainable use and conservation of paca populations.
In this study, we evaluated the effect of different variables (availability of food and cavities, interspecific competition, predation, and hunting pressure) on abundance and density of C. paca. In addition, we analyzed habitat use by pacas from a source-sink systems perspective in sites with three different land uses: Montes Azules Biosphere Reserve, community reserves, and agricultural landscapes of the Lacandon Rainforest. We expected that paca abundance and density would be positively associated to the availability of food and cavities, and negatively correlated to interspecific competition, predation, and hunting pressure. We predicted that pacas would prefer habitats with more food available, and cavities resistant to predation and hunting, which would favor their survival and reproductive success. Preferred (source) habitat types would show higher paca densities than the others.
Materials and Methods
Study area The study area included a southeastern portion of Montes Azules Biosphere Reserve (MABR) and territories of two neighboring communities (ejidos): Playón de la Gloria (pop = 209), and Reforma Agraria (pop = 145) in the Lacandon Rainforest of Chiapas, Mexico. Both communities are located on the east side of the Lacantun River (INE 2000; INEGI 2010). Three habitat types were considered in this study: 1) Transformed landscapes, including secondary vegetation along rivers and within agricultural and grazing areas, and human settlements. Human presence in this habitat type was continuous; 2) Community reserves, established by local residents for conservation in Reforma Agraria (14.6 km2) and Playon de la Gloria (2.5 km2). Both reserves are primarily covered by secondary forest, abandoned plantations, and rainforest fragments. Tourism and research are present in these reserves for relatively short periods (a month or less); 3) Montes Azules Reserve, where undisturbed mature rainforest is predominant. Human activity is negligible in this site, where only a few researches and visitors are allowed (Figure 1).

Figure 1 Location of the study site in the Lacandon Rainforest, Chiapas, Mexico. Transects (black lines) and habitat types are shown: Transformed landscape (light plain green), Community reserve (dark dotted green), and Montes Azules Biosphere Reserve (MABR; green with diagonal lines). Human settlements are represented with a white square: Reforma Agraria in the upper part, and Playon de la Gloria in the lower part.
Fieldwork. Monthly samplings were carried out in the study sites from September 2013 through August 2014. Four line transects were walked in each habitat type for each season (dry = December-May, wet = June-November). Each transect was 1 km long and 50 m wide (Figure 1). A minimum distance of 175 m between transects was set for sampling independence based on the average home range of the paca (1.74 ha; Beck-King et al. 1999). Our criterion to select transect locations was the presence of paca signs (footprints, feeding sites, active burrows, and sights by residents). Four camera-traps were deployed every 200 meters along each transect in order to estimate the relative abundance of pacas and their potential competitors and predators. That distance was considered appropriate for independence of paca records (Karanth and Nichols 1998) and for its competitors of similar or smaller size. For larger competitors and predators such as the white-tailed deer (Odocoileus virginianus), red brocket deer (Mazama temama), Baird's tapir (Tapirus bairdii), collared peccary (Pecari tajacu), white-lipped peccary (Tayassu pecari), jaguar (Panthera onca), puma (Puma concolor), ocelot (Leopardus pardalis), tayra (Eira barbara), and dogs (Canis lupus familiaris), the minimum distance for independent records was 1 km (Maffei and Noss 2008; Naranjo et al. 2015). Camera-traps (models Moultrie D55, Stealth-Cam Q8X, and Bush-nell Trophy XLT) were programmed to be active 24 hours for 30 consecutive days each season, taking 3 still pictures and a short video (10 to 20 sec) per event. Records were considered independent if taken in different transects and days, unless individual identification was possible (Monroy-Vilchis et al. 2011; Jax et al. 2015). Capture rates were estimated by calculating the number of independent records (pictures) of pacas in relation to the total number of effective records of all species per season and habitat type. Effective pictures were those where pacas or their potential competitors and predators appeared (Pérez 1992; Dubost and Henry 2006; Figueroa-de-León et al. 2016a).
Relative abundance indices of pacas and other mammals (Appendix 1) were estimated using photo-trapping data (RAIft). These indices were calculated as follows: RAIftv = number of independent records (pictures or videos) of C. paca / total effective pictures / sampling effort per 100 camera-days (Carbone et al. 2001). The density of pacas was assessed by counting the individuals photo-captured along each transect. Individuals recorded during abundance monitoring and photographic records previously obtained in cavities occupied by pacas (Figueroa-de-León et al. 2016a) were taken into account for density estimations. Individual identification was based on descriptions of pacas made by Figueroa-de-León et al. (2016a). Diffuse pictures were excluded from this analysis.
Food availability for pacas was evaluated by establishing five 1,000 m2 circular plots along each transect. The distance between centers of two contiguous plots was 200 m (Elzinga et al. 2001). All trees and palms within plots producing fruits potentially consumed by pacas (Beck-King et al. 1999; Muñoz et al. 2002; Zucaratto et al. 2010) were counted and marked. Field observations of fruits bitten by pacas and references by local hunters were also helpful for selecting the tree species considered in this analysis. Samples of leaves, flowers and fruits of each tree species were collected for taxonomic determination in the Herbarium at El Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas. Fruit production in each habitat type was monitored monthly between September 2013 and August 2014. Mean area under the tree crown and the average number of fallen fruits per tree were counted for each of the 30 tree species recorded. Fallen fruit counts were done monthly in 5-10 circular plots with a radius of 0.5 m (fruits up to 3 cm diameter) and 1 m (fruits over 3 cm diameter). All counted fruits were removed from the plots to avoid double counts in subsequent samplings. Collected fruits were taken to the bromatology lab at El Colegio de la Frontera Sur, where fruit parts were separated to estimate the biomass available as paca food (Beck-King et al. 1999; Muñoz et al. 2002; Zucaratto et al. 2010; Appendix 2). Fruit parts were dehydrated at 60 °C until constant weight (adapted from Wallace and Painter 2003). The numbers of fallen fruits per tree species were multiplied by the average dry weight of all parts consumed by pacas (Ganesh and Davidar 1999). Available food estimates from all tree species were pooled to obtain food availability indices by habitat type and season.
In April (dry season) and September (wet season) 2013 we intensively searched for cavities used as refuges by pacas. This search was done along each transect (1,000 x 50 m) from 7:00 through 16:00. All cavities found were examined, including those under the forest floor, within tree roots or fallen logs. Cavities were considered available for pacas if they were at least 15 cm in diameter, 60 cm deep (Aquino et al. 2012), and located at 100 m or less from the nearest water source (Figueroa-de-León et al. 2016a,b). Only cavities with indicators of recent activity (fresh tracks, fresh litter around, and clean entrance were considered. A minimum distance of 100 m between cavities was taken as a criterion for spatial independence (Beck-King et al. 1999). An index of cavity availability was estimated by dividing the number of available cavities by the total area sampled in each habitat type and season.
Hunting pressure on pacas was estimated by gathering information consisting of hunted paca carcasses found during fieldwork, paca cavities destroyed by hunters, pictures and videos of hunters and their dogs nearby cavities, platforms for hunting built on trees, personal observations of hunting by the authors, and comments by residents about the presence of paca hunters in their communities. A persistently hunted transect was that in which a hunting evidence was detected at least once a month, or when there were 2 to 3 weekly references about paca hunters present. A slightly hunted transect showed no frequent hunting evidence or weekly references. In addition, a hunting pressure index was built by dividing the number of persistently hunted transects by the number of slightly hunted transects in each habitat type and season. Habitat use by pacas was assessed by estimating their densities in the three habitat types and both seasons. The total available habitat was the sum of sampled areas in each transect, habitat type, and season (Morrison et al. 2006).
Data analyses. Paca abundance, density, food and cavity availability, and hunting pressure were compared among habitat types and between seasons using Wilcox-on's signed-rank tests, Kruskal-Wallis's analysis of variance on ranks tests, and Pearson's Chi-squared tests of independence with Yates's correction of continuity (Crawley 2005). Durbin and Watson's tests were used to verify independence in our abundance and density data (Crawley 2005). Generalized Linear Models (GLM; McCullagh and Nelder 1989) were built to determine the effect of explaining variables (food and cavity availability, hunting pressure, competition, and predation) on paca density and abundance indices. Because of our limited sample size (13 and 12 transects in dry and wet season, respectively), each model was built with a single explaining variable at a time. A Poisson distribution was assumed in the GLM unless an over-dispersion due to zero predominance in data was present. In such cases, a negative binomial distribution was assumed (Crawley 2005). All statistical procedures were applied using packages MASS (Venables and Ripley 2002), and lmtest (Zeileis and Hothorn 2002) in R platform version 3.3-1 (R Core Team 2016). HABUSE program (Neu et al. 1974; Byers et al. 1984) was used to assess habitat use based on paca abundance and its habitat availability. By running Chi-squared tests and Bonferroni's confidence intervals in this program, we detected preference or avoidance of each habitat type by pacas in the study area (Neu et al. 1974; Byers et al. 1984).
Results
A total of 125.3 km were walked and an area of 125 hectares was sampled in the study area. A sampling effort of 2,226 camera-days resulted in 5,745 photographic records of 23 mammal species. Of these records, 1,026 were considered effective photographs and 191 of them corresponded to C. paca. Paca relative abundance was slightly lower in the dry season than in the wet season (0.01 versus 0.02 photographs/100 camera-days, respectively). However, no significant differences were found between seasons (W = 77.5, P = 1) and among habitat types in dry (X2 = 0.2, P = 0.9) and wet (X2 = 2.8, P = 0.2) seasons. Paca densities were similar between seasons (dry = 0.56 pacas/ha, wet=0.54 pacas/ ha; W = 77.5, P = 0.99) and among habitat types (P > 0.05).
Food availability for pacas was estimated under the crown of 297 trees of 30 species, of which 5 species produced fruit in the dry season, 16 in the wet season, and 8 in both seasons. A lower food availability was found in the dry season compared to the wet season (W = 125, P = 0.009). Nonetheless, there were no differences among habitat types (P > 0.05; Table 1). Cavity availability was similar between seasons (W = 61.5, P = 0.4), and among habitat types (P > 0.05; Table 1). Similarly, we detected no seasonal (X2 = 0.04, P = 0.8) or habitat-based (P > 0.05; Table 1) differences in the numbers of persistently hunted versus slightly hunted transects in the study area.
Table 1 Relative abundance indices, densities, food and cavity availability indices, and hunting pressure on Cuniculus paca in three habitat types of the Lacandon Rainforest, Chiapas, Mexico (2013-2014). The following units were used: Relative abundance index (RAI): photographs/100 camera-days; density: pacas/ha; food availability index (FAI): kg/ ha; cavity availability index (CAI): number of cavities/ ha; hunting pressure index (HPI): number of persistently hunted transects/ number of slightly hunted transects.
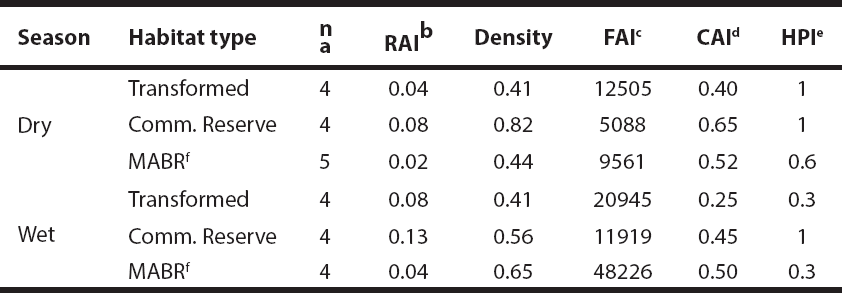
a Number of transects, b Relative abundance index: Number of photographic records of pacas / total effective photographs / days * 100. c Biomass (dry weight of edible parts of fallen fruits, kg) / ha of each habitat type. d Total cavities available / ha sampled in each habitat type. e Persistently hunted transects / slightly hunted transects. f MABR: Montes Azules Biosphere Reserve.
Potential paca competitors recorded by photo-trapping in MABR were: Red brocket deer, Baird's tapir, collared peccary, nine-banded armadillo (Dasypus novemcinctus), agouti (Dasyprocta punctata), opossums (Didelphis spp., Philander opossum, and Metachirus nudicaudatus), and great curassow (Craxrubra). In the community reserve we detected the white-tailed deer, red brocket deer, Baird's tapir, raccoon (Procyon lotor), coati (Nasua narica), nine-banded armadillo, opossums, great curassow, and green iguana (Iguanaiguana). In the transformed landscapes, the red brocket deer, Baird's tapir, collared peccary, and great curassow were registered. Potential predators detected through photo-trapping in MABR were the ocelot and tayra; the jaguar, puma, and dogs were recorded in the community reserve, and no predators were recorded in the transformed landscape.
Paca relative abundance was no significantly affected by food and cavity availability, hunting pressure or abundance of competitors and predators (Appendices 3 and 4). Contrastingly paca density was positively correlated with cavity availability (dry season: r = 0.15, P = 0.04; wet season: r = 0.22, P = 0.006). In the dry season, pacas used the transformed landscape in proportion to its availability, the community reserve more than expected, and MABR less than expected. However, during the wet season the three habitat types were used by pacas in proportion to their availability (Table 2).
Table 2 Habitat use and availability of Cuniculus paca in the Lacandon Rainforest, Chiapas, Mexico (2013-2014). HAB=Habitat type. Available habitat in the transformed landscape and the community reserve: 20 ha (dry and wet seasons). Available habitat in Montes Azules Biosphere Reserve (MABR): 25 ha (dry season), 20 ha (wet season).

aOBS: observed proportion of use. bEXP: expected proportion of use. cINTERVAL: Bonferroni's confidence intervals. dHU: habitat use by paca, where "=": used in proportion to availability; "+": used greater than expected (selected habitat); used lower than expected (P = 0.05).
Discussion
Similarities in paca abundances and densities between seasons and among habitat types may have been due to our limited sample sizes (13 and 12 transects in dry and wet seasons, respectively), which make difficult to detect potential differences. In addition, paca abundance indices may be skewed because differences in capture probabilities across seasons and habitat types were not considered. Capture probabilities are conditioned by factors such as individual behavior and physical condition, cover type around camera-traps, and weather, among others (O'Connell et al. 2010). These factors may be confounding potential effects of variables explaining paca abundance. Similarly, density estimates could have been subject to problems in distinguishing paca individuals because of low resolution of night pictures, and distance impairing the observation of the spot pattern of some pacas (Figueroa-de-León et al. 2016a). In spite of this, cavity availability was the only variable with a significant link with paca densities, which suggests that cavities constitute a key resource driving paca population dynamics in the study area.
Food availability did not show a significant effect on paca density and abundance. This can be partially explained by the methodological limitations mentioned above, or by the opportunistic behavior of pacas (Laska et al. 2003), which may adjust their diet following seasonal and spatial variations in fruit availability (Dubost and Henry 2006). Hunting pressure had no effect on paca density and abundance, which may be due to the relatively low frequency of hunting practices in the communities visited in this study. Yet, it is important to encourage multiannual surveys for better understanding the impact of hunting on paca population dynamics and its persistence in transformed landscapes.
The abundance of competitors did not have an effect on paca populations, probably because the ecological and behavioral traits of pacas (nocturnal activity, opportunist diet, and cavity use for avoiding predation; Aquino et al. 2009), allow them to reduce the encounter probability with other species. On the other side, the abundance of potential predators was unrelated to paca abundance. A likely explanation to this result is the fact that pacas reduce their predation risk by taking cover inside safe cavities such as those under tree roots or in fallen logs (Figueroa-de-León et al. 2016a, b), which are frequently located at strategic sites (i. e., near water courses that can be used as routes for escaping from predators; Aquino et al. 2009).
Pacas selectively used the community reserve of Playon de la Gloria during the dry season, probably due to the existence of a rocky area with multiple cavities of the appropriate size to avoid predation and hunting. In contrast, the three habitat types considered in this study were used in proportion to their availability during the wet season. This may be explained by: 1) An increase in paca movements favored by a shortage of cavities produced by seasonal floods or collapse of burrows; 2) food and water abundance. Proximity to permanent water sources has been documented as a key variable for selective use of cavities by pacas (Figueroa-de-León et al. 2016a). Similarly, water bodies have proven key habitat elements related to paca defecation (Figueroa-de León et al. 2016b) and reproduction (Epigmenio Cruz Aldán, Tuxtla Gutierrez Zoo, Chiapas, Mexico 2016 comm. pers.). Therefore, food and water abundance during the wet season reflects in lack of habitat selectivity by pacas in the study area.
In summary, we found that cavity availability had an effect on paca densities, which highlights the relevance of those habitat components on the population dynamics of this rodent in our study area. Besides, cavities used by pacas as shelters to avoid predation and adverse weather constitute habitat resources of utmost importance for pacas in the Lacandon Rainforest (Figueroa-de-León et al. 2016a). Thus, source habitats for pacas such as MABR and the community reserve of Playon de la Gloria have high availability of cavities favoring higher paca densities and reproductive rates inferred from higher frequencies of newborns (Figueroa-de-León et al. 2016a). On the contrary, sink habitats for pacas such as the transformed landscape included in this study have lower availability of appropriate cavities, lower densities and absence of newborns (Figueroa-de-León et al. 2016a).
Conserving wildlife species tolerant to land use change (i. e., the paca) in transformed landscapes will depend on their habitat requirements as well as the distribution and connectivity of habitat fragments available (Ye et al. 2013). Hence, habitat types with continuous, mature cover are as important for paca conservation and management as secondary forest fragments interconnected to the first (Jax et al. 2015). Habitat connectivity is fundamental for paca mobility and dispersal, improving the viability of its populations in the long term (Ahumada et al. 2003; Gallina et al. 2012).











 text new page (beta)
text new page (beta)


