Introduction
Frugivorous or omnivorous mammals are a key component in tropical forest ecosystems, since they serve as seed dispersers or predators (O'Farrill et al. 2006, 2011; Peres et al. 2016). This contributes to the regeneration of forests, by structuring their composition (Peres et al. 2016). However, the reduction of tropical forests through changes in land use affects the diversity and availability of fruits and prolongs the natural periods of scarcity (Tabarelli et ai. 2004; Keuroghlian and Eaton 2008). As a result, some mammal species migrate to other areas in search of fruits, while others are able to make adjustments to their diets (Altricher et al. 2001; Keuroghlian 2003; Keuroghlian and Eaton 2008). In peccary species (Tayassu pecari and Dicotyles tajacu), it has been pointed out that the breeding, gestation and birth periods are related to the nutritional quality of food resources and the primary productivity of key fruit species available in the environment (Altricher et al. 2001; Keuroghlian et al. 2004; López et al. 2006).
The white-lipped peccary (T. pecari) and the collared peccary (D. tajacu), belonging to the Family Tayassuidae, are important social ungulate species in tropical ecosystems. Both species contribute to the maintenance and composition of trees in forests through herbivory and seed dispersal and predation (Bodmer 1991; March 1993; Beck 2005,2006; Keuroghlian and Eaton 2009; Beck et al. 2010). Peccaries are an essential food resource for the inhabitants of rural and indigenous communities throughout their distribution range (Weber 2000; Altrichter and Boaglio 2004; Desbiez et al. 2009; Reyna-Hurtado et al. 2010; Briceño-Méndez et al. 2011; Keuroghlian et al. 2013; Naranjo et al. 2015). Wild populations of both species are currently under an intense pressure by hunting and loss of habitat (Reyna-Hurtado 2009; Góngora et al. 2011; Altrichter et al. 2012; Keuroghlian et al. 2013; Naranjo et al. 2015; Briceño-Méndez etal. 2016).
The Calakmul region, in the state of Campeche, is one of the main remnants of tropical forest in Mexico and includes the Calakmul Biosphere Reserve (RBC; 7,231 km2). Various activities such as the production of charcoal and the use of natural resources have led the accelerated deforestation and fragmentation of the primary vegetation, in addition to poaching of wild species. A site adjacent to this reserve is the ejido Nuevo Becal, stretching across 520 km2, where the loss of habitat and poaching of wild species prevail (Briceño-Méndez et al. 2016). The forests of the Calakmul region include two tree species that are key in the diet of peccaries (Reyna-Hurtado 2007; Perez-Cortez and Reyna-Hurtado 2008): the ramon, Brosimum alicastrum, and the zapote, Manilkara zapota. These species account for 57.10 % of the total species producing fruits consumed by peccaries on the ground (Briceño-Méndez et al. 2014). Given the importance of their fruits as a source of food, these species have also been described as part of the diet of primates such as the spider monkey, Ateles geoffroyi, and the howler monkey, Alouatta pigra (Hernández-Sarabia 2013), as well as of other ungulates such as the tapir, Tapirus bairdii (O'Farrill et al. 2006, 2011) and the temazates deer, Mazama temama and M. pandora (Weber 2008; González-Zamora et al. 2009).
An investigation conducted in RBC has revealed that the availability of ramon is related to the surface area used by T. pecari (Reyna-Hurtado 2007). However, it is unknown whether the proportion of newborns in groups of peccaries varies according to the season. This could be related to the availability of M. zapota and B. alicastrum fruits, documented as essential seasonal food items in the peccary diet (Reyna-Hurtado 2007, Perez-Cortez and Reyna-Hurtado 2008). Unveiling the relationship between the availability of key fruit species such as ramón and zapote and the composition in the social structure of peccaries is essential for management and conservation plans. The objectives of this study were two. Quantify the seasonal availability and variations of M. zapota and B. alicastrum fruits in ejido Nuevo Becal. Evaluate the social structure of T. pecari and D. tajacu. Specifically, it was assessed whether there are fluctuations in the number of newborns of the two peccary species according to the rainy and dry seasons matching the availability of M. zapota and B. alicastrum fruits.
Materials and Methods
Area of study. The site is located in the southeastern portion of the Calakmul region, at ejido Nuevo Becal (18.6920° N, -89.2511° W; 20.9450° N, -89.6433° N; 21.2811° N, -89.6650° W; 21.0161° N, -89.8772° W), in the municipality of Calakmul, Campeche, Mexico. This area is adjacent to RBC, a protected area of tropical forest in Mexico with an extension of 7,231 km2 (Figure 1).

Figure 1 Location of the study area and sampling points at ejido Nuevo Becal, Calakmul, Campeche Mexico.
The ejido comprises an area of 520 km2. The types of vegetation include subdeciduous forest, floodplain forests, dry forests, and secondary vegetation (Pennington and Sarukhan 1998). Elevation ranges between 100 to 380 masl. The predominant climate is warm sub-humid with summer rainfall and with less than 60 mm of precipitation in the driest month; the mean annual temperature is 25 °C (García-Gil 2003).
Social structure. During the 2014 dry season (February-May) and the 2015 rainy season (June-September) peccary groups were monitored in 10 camera-trap stations (Reconyx PC800 Hyperfire Professional IRTM y PC600 Hyperfire Pro White FlashTM; Reconyx, Inc., Holmen, Wisconsin, USA) on a permanent basis in sites near water bodies, roads and trails selected at random in the ejido section covered by natural vegetation. Camera traps were placed at a height not exceeding 50 cm from ground level and with a separation of 1.5 km, covering an area of approximately 112 km2 delimited by the external location of traps (Figure 1). The period of photographic records was set to operate 24 hours with trigger intervals of one second. This interval allows counting all individuals passing in a line or grouped (Maffei et al. 2002; Figure 2). Records were considered independent after 24 hours between one record and another, or when more than one individual appeared in the photograph. The position of each station was georeferenced with a Garmin 62s® GPS. For each picture obtained, the time and date was recorded (Lira Torres et al. 2014). The age structure of each group was examined and evaluated by obtaining the individuals into three categories, adults, juveniles, and young, according to size and pelage coloration; then, the percentage of each category was calculated (Reyna-Hurtado et al. 2010). The average number of hatchlings was estimated and counted during the month of birth, either in the February-May dry season or in the June-September rainy season (Figure 2).

Figure 2 Group of white-lipped peccaries (Tayassu pecari) photographed with camera traps. Blue arrows indicate five newborns of white-lipped peccaries in ejido Nuevo Becal, Calakmul, Campeche, Mexico.
Fruit Availability. To estimate fruit abundance, five 2 km-long transects were established, which were determined randomly in forested areas, avoiding a radius of at least 7 km from the village. Transects were visited once a month in order to derive a fruit abundance index (Altrichter et al. 2001). The method consists in finding a fruit on the ground, then locating the source tree where the fruit came from, provided it is located at a perpendicular distance not exceeding 5 m from the center line of the transect. Once the source tree was located, all the fruits found within a quadrat of 2 m2 under the canopy were recorded.
Data Analysis. To evaluate the differences in the size of the groups of both species between the dry and rainy seasons, and the proportion newborns, Mann-Whitney tests were conducted. We used the availability index of each fruit species and the monthly variation was evaluated using a Pearson's Chi2 non-parametric test. In all cases, statistical tests considered a significance level of P < 0.05. The variables analyzed were the proportion of newborns per group, species, fruit availability index, dry season and rainy season. A simple correlation analysis was conducted between fruit availability index values and number of newborns. The statistical analyzes were performed in SPSS v. 17.0.
Results
The sampling effort was 2,420 trap-nights (302.5 trap-nights per month) and a total of 86 records of Tayassu pecari were obtained, resulting in a total of 1,026 individuals counted. For Dicotyles tajacu, 76 records were obtained, leading to a total of 33 individuals counted.
A higher number of groups and individuals of both species were observed in the dry season (T. pecari 53.8; D. tajacu 46.2) versus the rainy season (T. Pecari 33.1; D. tajacu 30.1). Similarly, the average size (mean ± SD) of groups for both species was significantly higher in the dry season (T. pecari 23 ± 5.3, n = 53; D. tajacu 4.9 ± 2.6, n = 46; U = 0.01).
Social structure. The age structure of T. pecari in the dry season was determined based on 886 individuals, with the following proportions: adults, 76.3 %; sub-adults, 9.4 %; and newborns 14.3 %. In the rainy season, the age structure on the basis of 140 adult individuals was 66.6 %, sub-adults 26.2 % and 7.2 % newborns. The age structure of D. tajacu in the dry season (n = 46) was: adults, 76.0 %; sub-adults, 7.7 %; and newborns, 16.3 %; the percentages of these age classes for the rainy season (n = 17) are 93.0 %, 6.0 % and 1.0 %, respectively. In both species, the proportion of offspring was significantly higher during the months of the dry season T. pecari (X2 = 27.14, d. f. = 7, P < 0.001) and D. tajacu (X2 = 34.0, d. f. = 5, P < 0.001, Figure 3, 4).

Figure 3 Proportion of the number of newborns in groups of white-lipped peccary (Tayassu pecari) (blue bars), and collared peccary (Dicotyles tajacu) ( yellow bars) recorded during the 2014 dry season and 2015 rainy season in ejido Nuevo Becal, Calakmul, Campeche, Mexico.
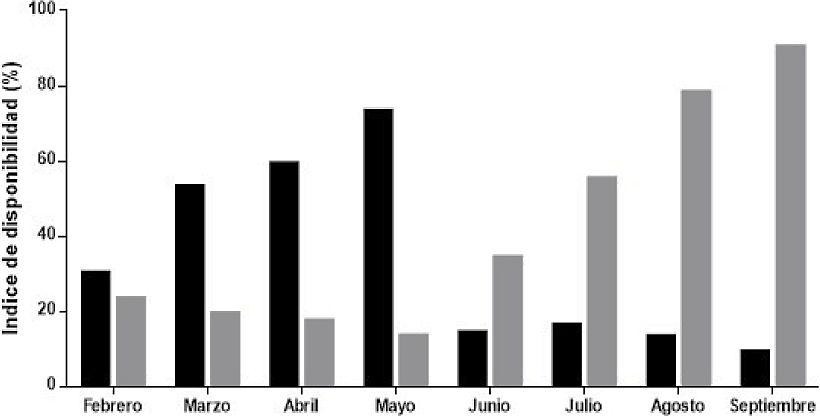
Figure 4 Availability of fruits of zapote Manilkara zapota (black bars) and ramon Brosimun alicastrum (gray bars) during the dry season (Feb-May 2014) and rainy season (June-Sept 2015) in ejido Nuevo Becal, Calakmul, Campeche, Mexico.
Fruit Availability. The walkthrough of 80 km of transects (n = 5) in 15 plots per season (dry and rainy seasons) yielded a total of 329 fruits of B. alicastrum and 267 of M. zapota. The fruit availability index for ramon was variable, peaking in September (X2 = 39.00, d. f. = 3, P < 0.001, Figure 4). The availability of M. zapota fruits was significantly higher in the dry season and varied throughout this season, reaching its peak availability in May (X2 = 33.14, d. f. = 7, P < 0.001, Figure 4). The proportion of offspring in both peccary species was significantly correlated with the availability of M. zapota fruits during the dry season (T. pecari, r2 = 0.43; D. tajacu, r2 = 0.46).
Discussion and Conclusions
Social Structure and Fruit Availability. In both species, the social structure within groups showed a higher proportion of adults. This is consistent with the findings reported in the Calakmul Biosphere Reserve (Reyna-Hurtado et al. 2010), in areas subjected to hunting pressure in ejido Nuevo Becal for the white-lipped peccary (Briceño-Méndez et al. 2016), and for the collared peccary in the Chimalapas, Oaxaca (Pérez Irineo and Santos Moreno 2016).
The proportion of offspring was higher in the dry season than in the rainy season for both species; this is consistent with the findings reported for white-lipped peccaries in the Calakmul Biosphere Reserve (Reyna-Hurtado et al. 2010). However, for the collared peccary these findings are contrary to those reported in French Guiana, where the proportion of offspring is higher in the rainy season (Henry 1994).
Although there are no data on the nutritional value of the fruit species for peccaries, it has been reported that both ramon and zapote play a key role in the seasonal diet of both species (Perez-Cortez and Reyna-Hurtado 2008). This fact may explain the relationship between the presence of a higher number of offspring of both species during the dry season and the fruit abundance index for zapote fruits. Two plant species (Ficus spp. and Licania operculipetata) have been mentoned as fructifying during periods of shortage of other food items, and are related to the breeding season and number of offspring, which affects the size and composition of groups in both peccary species, although not necessarily being the factor that could be driving the increase in group size in both species (Altricher et al. 2001; Keuroghlian 2004). Another possible explanation in relation to the number of offspring during the greater availability of fruits, is that these may be providing postpartum mothers nutrient needs for young infants (Lopez et al. 2006).
The fruits of Pouteria campechiana, Ampelocera hottlei, Cratavea tapia, Byrsonima crassifolia, Citrullus vulgaris, Talisia olivaeformis and Metopium brownei are identified as potential food items for peccaries in the region, being a supplement to their diet (Perez-Cortez and Reyna-Hurtado 2008). The production of these fruits is variable; they are usually available for very short periods of time (Reyna-Hurtado 2007; Briceño-Méndez et al. 2014), contrary to the zapote and ramon fruits, which are available throught a season. For example, zapote can reach peak fruit availability in the dry season during May, while ramon reaches its highest fruit abundance index values in the rainy season (Reyna-Hurtado 2007; Briceño-Méndez et al. 2014).
Both peccary species are benefited by the availability and search for these important dietary resources in heterogeneous environments during well-marked seasons of the year in the study site. In addition, peccaries can consume a wide variety of food types available in the landscape, and even modify their diet (Keuroghlian 2004; Beck 2005; Keuroghlian and Eaton 2008; Fernandes et al. 2013).
Our results reveal the relationship between the primary productivity of fruit species and the size and social composition in groups of both peccary species in the tropics (Altrichter et al. 2001). There are other factors related to group composition and size; for example, it has been documented that in the rainy season white-lipped peccaries consume small amounts of animal food items, including invertebrates and some fish species (Fernandes et al. 2013; Reyna-Hurtado 2007). Water availability, the state of conservation of the habitat and the hunting pressure have been considered as factors that strongly influence group size in peccaries inhabiting the Calakmul region (Reyna-Hurtado et al. 2015).
Conservation Perspectives. An important factor for the conservation of peccaries is the reduction and/or control of zapote tree logging, which is an important resource in the study site. The plant species consumed by peccaries are also relevant for other endangered species such as the spider monkey (Ateles geoffroyi), howler monkey (Alouatta pigra), tapir (Tapirus bairdii), temazate deer (Mazama temama and Mazama pandora), all of which depend to a large extent on these fruit species to supplement their diet (O'Farrill et al. 2006, 2011; Weber 2008; González-Zamora et al. 2009).
The ejido Nuevo Becal is home to endangered species and includes habitats in good state of conservation (Briceño Méndez et al. 2017). However, human activities such as hunting and loss of habitat still prevail (Escamilla et al. 2000; Santos Fita et al. 2012; Briceño-Méndez et al. 2014). Therefore, it is imperative to introduce feasible subsistence strategies to the local community (Montiel et al. 1999), particularly for being a rural community that is connected to the Calakmul Biosphere Reserve.











 nova página do texto(beta)
nova página do texto(beta)


