Introduction
The water opossum or Yapok, Chironectes minimus (Zimmerman 1780), is the only Neotropical semi-aquatic marsupial (Bressiani and Graipel 2008; Acosta and Azurduy 2009; Galliez et al. 2009). It belongs to a monotypic genus, which includes four subspecies (Stein and Patton 2007; Damasceno and Astúa 2016). The species is characterized by a silvery gray dorsal pelage with four black transverse patches connected by a narrow midline. Water opossums are adapted to semi-aquatic habitats, with several external morphological adaptations: 1) dense, short, and water-resistant pelage, 2) webbed hindfeet to swim, 3) impermeable pouch in females to keep the young dry, and 4) the ability to protect the male genitalia in the water with an incomplete pouch (Mondolfi and Medina 1957; Marshall 1978; Stein and Patton 2007; Voss and Jansa 2009).
Water opossums are widely distributed in the Neotropics (Figure 1), from southern Mexico to northeastern Argentina (Nowak 1999; Cuarón et al. 2008). This elusive and solitary species is mainly associated with river channels with stony substrates, clear and fast-running waters, and preserved riparian vegetation (Prieto-Torres et al. 2008; Galliez et al. 2009; Galliez and Fernandez 2012; Ardente et al. 2013). However, it is a species whose large-scale population dynamics (e. g., distribution and abundance) cannot be studied using traditional methods, because they are not usually captured in common live traps for small mammals (Bressiani and Graipel 2008; Prieto-Torres et al. 2011). In fact, although there are some studies on the behavior, demographic patterns, habitat selection, morpho-physiological and genetic analyses of water opossums (e. g., Nogueira et al. 2004; Galliez et al. 2009; Palmeirim et al. 2014; Fernandez et al. 2015), most of them are not-specific, faunistic surveys (e. g., Handley 1976; Oliveira et al. 2007; Prieto-Torres et al. 2008; 2011; Ardente et al. 2013).
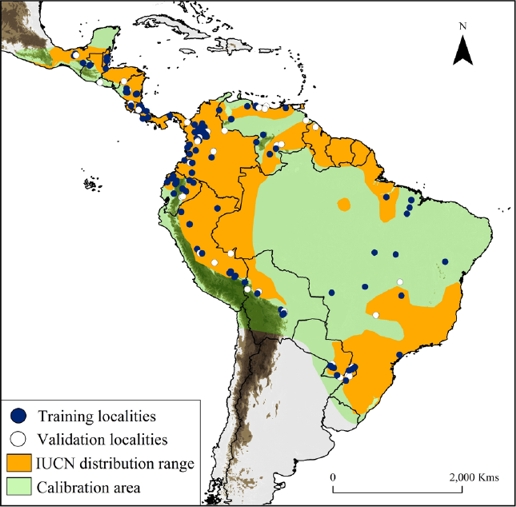
Figure 1 Map showing water opossum (Chironectes minimus) unique records (n = 165), overlaid with the IUCN distribution and model calibration area (light and dark green colors). Training localities (blue dots) and validation localities (white dots) were used to generate and validate the models. Dark brown color represents area with altitudes of up 1,200 m.
The water opossum is listed as Least Concern (Cuarón et al. 2008) on the International Union for Conservation of Nature (IUCN) red list due to its wide distribution, presumably large population, and its presence in several protected areas or “PAs” (Oliveira et al. 2007; Galliez et al. 2009; Ardente et al. 2013). However, recent work suggests a decreasing population trend in Brazil, where the species is considered threatened in at least five states due to habitat loss and degradation (Ardente et al. 2013; Palmeirim et al. 2014; Fernandez et al. 2015). Thus, there is an increasing need to define its actual distribution and ecological requirements (Cuarón et al. 2008).
The minimum convex polygon method is frequently used to estimate species’ distribution (IUCN 2001, 2015), but ignores the species’ ecological constraints (Brown et al. 1996; Mota-Vargas and Rojas-Soto 2012; Peterson et al. 2016). Thus, techniques like species distribution models (SDMs) have been developed to predict the potential distribution of a species, identifying the suitable areas and the most important variables for the persistence of the species (Peterson 2001; Soberón and Peterson 2005; Stohlgren et al. 2011). These models are widely used in ecology, evolution, conservation, and management (e. g., Soberón and Peterson 2005; Stohlgren et al. 2011; Tôrres et al. 2012; Ortega-Andrade et al. 2013; 2015).
Due to the lack of information on the distribution of C. minimus, in this study we modeled its potential distribution on a continental scale, following part of the methodology of Rheingantz et al. (2014) employed for another semi-aquatic mammal. We determined the effect of habitat loss in the extents of habitat suitability for species and evaluated if the current PAs systems actually harbor the most suitable environmental conditions for its distribution. Finally, we identified gaps in the potential distribution where future survey efforts and ecological studies should be focused.
Material and Methods
Collection of historical records. We compiled a database of occurrences from three sources: 1) occurrences available in on-line databases (i. e., Global Biodiversity Information Facility database [GBIF] and Mammal Networked Information System [MaNIS]); 2) specimens verified from biological collections (see Appendix 1); and 3) location records obtained from fieldwork and published literature (e. g., Handley 1976; Mares et al. 1986; Oliveira et al. 2007; Bressiani and Graipel 2008; Prieto-Torres et al. 2008; 2011; Acosta and Azurduy 2009; Ardente et al. 2013; Brandão et al. 2014; Damasceno and Astúa 2016). We verified each locality using Google Earth and MapLink (http://www.maplink.com), correcting imprecise coordinates and/or eliminating duplicates when necessary. Geographic coordinates were provided in decimal degrees, based on the WGS 84 datum. We obtained data from sixteen countries between 1925 and 2015 describing the historical presence of the species (Figure 1, Appendix 1). In addition, we considered the largest water opossum home range (~3 km2; Galliez et al. 2009) as a buffer area between records and cleared the points located close together, thereby reducing sampling bias (e. g., Ortega-Andrade et al. 2015). We performed the SDM (see below) using 165 unique localities records (Appendix 1).
Species Distribution Model and validation. We modeled the potential water opossum distribution with MaxEnt version 3.3.3k (Peterson 2001; Elith et al. 2006; Phillips et al. 2006), which uses the principle of maximum entropy to calculate the most likely distribution of the focal species in function of occurrence localities and environmental variables. We used the 19 climatic variables of WorldClim 1.4 (Hijmans et al. 2005) and three topographic variables (i. e., Digital Elevation Model [DEM], Slope and Aspect) from the Hydro 1K project (USGS 2001); with 30” of resolution (~1 km2 cell size). Despite that topographic variables are not commonly used in SDM studies, they were included because numerous examples (e. g., Mota-Vargas et al. 2013; Cauwer et al. 2014; Rheingantz et al. 2014; Kübler et al. 2016) show that these variables can be used as proxies for variables (e. g., micro-climate or edaphic conditions) that are correlated with physiological requirements of species.
The potential distribution model was generated using the 75 % (n = 124) of the locality records and 25 % (n = 41) for internal evaluation. In this sense, the algorithm used localities of species records and environmental conditions to perform a certain number of iterations (1,000 in this case) before reaching a convergence limit. This algorithm for the logistic output produces a map of habitat suitability ranging from 0 (unsuitable) to 1 (perfectly adequate; Phillips et al. 2006; Phillips and Dubik 2008). We ran ten cross-validate replicates to calculate confidence intervals, and the best model was selected based on the performance of area under the curve or “AUC” (Elith et al. 2006; 2011). Then, we converted the obtained logistic values of suitability rating into a binary presence-absence map, based on two established threshold values: the “Fixed cumulative value 10” (FCV10) and the “5 percentile training presence” (5PTP; see Pearson et al. 2006; Liu et al. 2013).
It is important to note that there is no rule to set these thresholds because its selection depends on the data used or the objective of the map, and will vary from species to species. In our case, we used the FCV10 as we wanted a threshold that minimizes the commission errors in our final binary maps (Liu et al. 2013), and we used the 5PTP to identify pixels with the highest suitability values, rejecting the lowest (5 %) suitability values of training records. The 5PTP model is a sub-conjunct in the geographic and ecological space of FCV10 model.
Given that ENMs do not address the historical aspects relating to species distribution (e. g., accessibility or “M” sensu BAM diagram [Soberón and Peterson 2005]), we used a geographical clip (Figure 1; Appendix 2) based on the intersection of Terrestrial Ecoregions (Olson et al. 2001) and the Biogeographical Provinces of the Neotropic (Morrone 2014) to create an area for model calibration (see Anderson and Raza 2010; Barve et al. 2011; Rodda et al. 2011). We selected the uncorrelated (r < 0.8) and most relevant variables using the Jackknife test of MaxEnt (Royle et al. 2012). These steps allowed us to reduce over-fitting of the generated suitability models (Peterson et al. 2011). Finally, we evaluated the performance of the selected MaxEnt model with the Partial-ROC (Receiver Operating Characteristic) curves test (Lobo et al. 2008). This criterion was used to solve problems associated with an inappropriate weighting of the omission and commission errors during the AUC analysis (see Lobo et al. 2008; Peterson et al. 2008).
Spatial analysis of the water opossum’ distribution in the Neotropics. We performed three distinct spatial analyses to assess the conservation issues related to the species’ potential distribution: 1) to evaluate the extent of habitat loss on the model; 2) to determine if the PAs system contains the highly suitable areas for the species; and 3) to identify the gaps where future survey efforts should be focused. The spatial analyses and map algebra were carried out with ArcMap 10.2.2 software (ESRI 2011), with a grid cell resolution of 30’’, corresponding to ~1 km2 in each raster.
First, we used a vegetation land cover map (Hansen et al. 2013) considering only two categories “natural forest” and “perturbed areas,” to determine the effect of habitat loss in the obtained models. Perturbed areas included urban areas, deforested areas, farm lands, and pastures for cattle ranching (Hansen et al. 2013). The PAs extents were downloaded from ProtectedPlanet.net (IUCN and UNEP-WCMC 2012). To assess if the current PA system harbors the most suitable environmental conditions for the species we performed a Kolmogorov-Smirnov (KS) test in R (R-Core-Team 2012) comparing the suitability values within and outside PAs (Rheingantz et al. 2014). The results obtained from the deforestation and PAs analysis were compared with the IUCN species distribution.
Finally, to identify gaps in the potential distribution where future survey efforts and conservation initiatives should be focused, we followed the proposal by Rheingantz et al. (2014). In the analysis, we multiplied the suitability value of a pixel by its distance to the nearest occurrence and river, based on the assumption that ecological similarities decrease with distance among these factors. Then, we divided the index by its highest value to obtain a scale from 0 to 1. We therefore assumed that areas with high suitability values, located far from previous studies and near to rivers (the focal species is associated with water) were more likely to be in different ecosystems or to have dissimilar environmental characteristics (Rheingantz et al. 2014). Thus, studying water opossum in those areas could explain whether the species uses different habitats than previously reported.
Results
Historical records and SDM for water opossum. Our study includes new information on the distribution of water opossum, including a total of 292 occurrences in the 16 countries that encompass the recognized distribution ranges according to the IUCN (Figure 1; Marshall 1978; Cuarón et al. 2008). Including also new potential areas of distribution in Mexico, El Salvador, Nicaragua, Costa Rica, Colombia, Venezuela, Brazil, Bolivia, Peru and Ecuador.
The variables used and their percentage contribution to the model are shown in the Table 1 and are consistent with results found by previous studies on Neotropical mammals (e. g., DeMatteo and Loiselle 2008; Tôrres et al. 2012; Rheingantz et al. 2014). We generated a model for water opossum distribution with a high Roc-Partial result (1.23 ± 0.09; P < 0.05). For the threshold FCV10 (0.160) and 5PTP (0.190), based on 41 test occurrences, we obtained 7 % (n = 3) and 5 % (n = 2) rates of omission, respectively. Performance assessment showed that models were statistically acceptable to describe the ecological niche and distribution of this species.
Table 1 Summary of the selected environmental variables with relative contributions (%) to the model of Chironectes minimus using MaxEnt 3.3.3k
| Abbreviation | Environmental Variable | Percentage contribution |
|---|---|---|
| Bio 18 | Precipitation of Warmest Quarter | 24.7 |
| Bio 11 | Mean Temperature of Coldest Quarter | 17.2 |
| DEM | Digital Elevation Model | 16.5 |
| Bio 07 | Temperature Annual Range (BIO5-BIO6) | 13.1 |
| Bio 14 | Precipitation of Driest Month | 12.9 |
| Bio 04 | Temperature Seasonality (standard deviation *100) | 7.8 |
| Bio 15 | Precipitation Seasonality (Coefficient of Variation) | 5.4 |
| Bio 01 | Annual Mean Temperature | 1.4 |
| Bio 03 | Isothermality (BIO2/BIO7) (* 100) | 0.9 |
The water opossum potential distribution according to the FCV10 threshold totaled ~9,238,000 km2, representing 45.9 % of the total areas used in the calibration of the model (Figure 2a). This FCV10 model is ~23 % wider than IUCN’s historical distribution map (with ~72.29 % overlap). Considering the 5PTP threshold, we obtained ~7,787,700 km2 of potential distribution for the species, representing 38.3 % of calibration areas and is ~4 % greater than the IUCN’s distribution map (with ~65 % overlap). The 5PTP’s potential species distribution was smaller in almost all countries compared to the IUCN map (Figure 2a). Comparing the IUCN map and FCV10 threshold, the only regions absent in the latter were predominantly areas in Mexico, savanna in Colombia and Venezuela, amazon in Peru, and the southeast of Brazil.
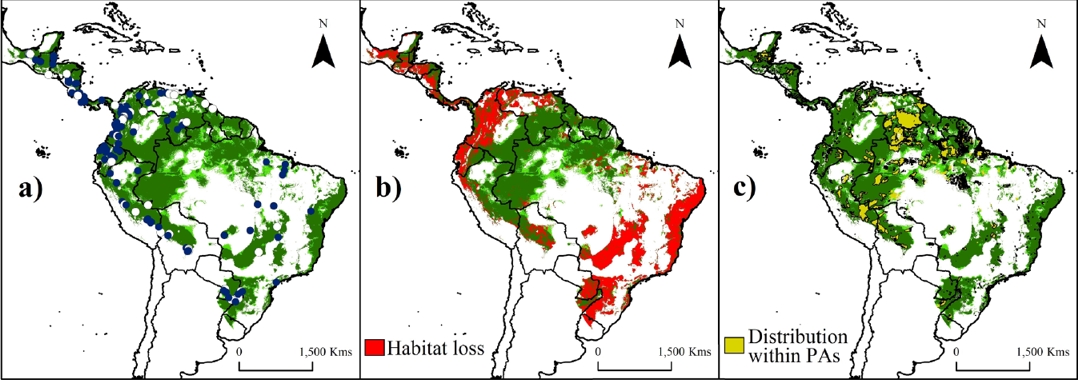
Figure 2 Potential suitability areas (a), remnant of natural forests (b) and predicted Protected Areas (c) throughout the distribution range of water opossum (Chironectes minimus). Training localities (blue dots) and validation localities (white dots) used to generate models are shown in (a). Potential distribution model (in a-c) is shown with the threshold value of Fixed cumulative value 10 (FCV10, light green) and 5 Percentile training presence (5PTP; dark green). Note an important reduction (~40 %, in greens [natural forests areas]) in the potential distribution model through the Mesoamerican region (from Mexico to Panama), the lowlands of the Andes region (from Peru to Colombia and northwest Venezuela), and the southeast of South America (Paraguay, Argentina and Brazil). The perturbed areas were calculated according the deforestation index map proposed by Hansen et al. (2013). Dark brown color represents area with altitudes of up 1,200 m.
Deforestation impact, protected areas and future areas of study. The predicted and remnant areas of the potential distribution model for the water opossum according the threshold values are detailed in Tables 2 and 3. Deforestation reduced the area of suitable water opossum habitat by ~40 % (38.07 – 43.39 %). Loss in area was most pronounced in the Mesoamerican region (from Mexico to Panama), the lowlands of the Andes region (from Peru to Colombia and northwest Venezuela), and the southeast region of South America (Paraguay, Argentina and Brazil; Figure 2b). Furthermore, only ~18 % of the potential water opossum distribution corresponds to natural forest within PAs (Figure 2b-c; Tables 2-3).
Table 2 Potential distribution models for Chironectes minimus, with percentage loss of potential distribution areas by effect of habitat loss and the percentage of potential distribution within Protected Areas (PAs) in the Neotropics
| Model | Area (~km2) | % |
|---|---|---|
| Extent of occurrence (minimum convex polygon) | 13,878,685 | - |
| IUCN distribution map | 7,501,124 | 100.00 |
| Area of the model within natural forests | 4,246,209 | 56.61 |
| Area of the model within PAs | 1,126,857 | 15.02 |
| Remnant model within PAs and natural forests | 978,935 | 13.05 |
| Species Distribution Model (FCV10) | 9,238,072 | 100.00 |
| Area of the model within natural forests | 5,721,975 | 61.93 |
| Area of the model within PAs | 1,840,152 | 19.91 |
| Remnant model within PAs and natural forests | 1,644,964 | 17.81 |
| Species Distribution Model (5PTP) | 7,787,759 | 100.00 |
| Area of the model within natural forests | 4,726,649 | 60.69 |
| Area of the model within PAs | 1,547,148 | 19.87 |
| Remnant model within PAs and natural forests | 1,381,237 | 17.74 |
Table 3 Potential distribution of water opossum (Chironectes minimus) estimated by country. Potential distributions are in km2 and percentages for each country, considering the deforestation effects and PAs, based in the two threshold values used in this study.
| Country | FCV10 | 5PTP | ||||
|---|---|---|---|---|---|---|
| Modeled Area (%) | Intact Areas (%) | Intact areas in PAs (%) | Modeled Area (%) | Intact Areas (%) | Intact areas in PAs (%) | |
| Brazil | 4,555,022 (49.31) | 2,642,873 (28.61) | 774,870 (8.39) | 3,604,227 (46.28) | 1,980,563 (25.43) | 569,951 (7.32) |
| Colombia | 1,042,751 (11.28) | 569,586 (6.16) | 58,024 (0.63) | 953,324 (12.24) | 523,079 (6.72) | 56,206 (0.72) |
| Venezuela | 831,292 (8.99) | 590,105 (6.38) | 383,794 (4.15) | 741,999 (9.53) | 549,763 (7.06) | 366,851 (4.71) |
| Peru | 744,347 (8.06) | 653,448 (7.07) | 136,241 (1.47) | 651,262 (8.36) | 567,470 (7.28) | 124,656 (1.60) |
| Bolivia | 383,783 (4.15) | 270,766 (2.93) | 77,297 (0.84) | 321,390 (4.12) | 220,288 (2.83) | 66,861 (0.86) |
| Ecuador | 248,756 (2.69) | 117,683 (1.27) | 31,479 (0.34) | 239,138 (3.07) | 113,795 (1.46) | 30,714 (0.39) |
| Guyana | 211,967(2.29) | 201,563 (2.18) | 19,638 (0.21) | 172,955 (2.22) | 163,320 (2.09) | 17,797 (0.23) |
| Mexico | 206,711 (2.24) | 91,310 (0.98) | 20,817 (0.22) | 183,380 (2.35) | 83,238 (1.06) | 17,907 (0.23) |
| Suriname | 155,010 (1.68) | 150,754 (1.63) | 16,497 (0.18) | 126,002 (1.62) | 122,293 (1.57) | 10,965 (0.14) |
| Paraguay | 154,595 (1.67) | 41,180 (0.44) | 2,809 (0.03) | 140,937 (1.81) | 36,895 (0.47) | 2,803 (0.04) |
| Nicaragua | 114,767 (1.24) | 64,038 (0.69) | 12,556 (0.14) | 112,145 (1.44) | 63,173 (0.81) | 12,546 (0.16) |
| Honduras | 112,529 (1.22) | 44,931 (0.48) | 6,798 (0.07) | 108,749 (1.39) | 44,418 (0.57) | 6,650 (0.08) |
| Guatemala | 108,169 (1.17) | 53,141 (0.57) | 22,504 (0.24) | 103,319 (1.33) | 50,843 (0.65) | 21,417 (0.27) |
| Argentina | 102,436 (1.11) | 65,902 (0.71) | 17,084 (0.18) | 90,662 (1.16) | 58,660 (0.75) | 16,630 (0.21) |
| French Guiana | 79,829 (0.86) | 79,018 (0.85) | 38,863 (0.42) | 67,426 (0.86) | 66,917 (0.86) | 33,904 (0.43) |
| Panama | 73,839 (0.79) | 38,500 (0.41) | 8,544 (0.09) | 67,499 (0.87) | 36,004 (0.46) | 8,284 (0.11) |
| Costa Rica | 49,732 (0.54) | 23,037 (0.25) | 8,452 (0.09) | 47,761 (0.61) | 22,519 (0.29) | 8,398 (0.11) |
| Uruguay | 30,447 (0.33) | 1,642 (0.017) | 207 (0.002) | 24,400 (0.31) | 1,063 (0.013) | 207 (0.002) |
| Belize | 24,201 (0.26) | 19,666 (0.21) | 8,248 (0.09) | 24,200 (0.31) | 19,666 (0.25) | 8,248 (0.11) |
| Trinidad and Tobago | 4,909 (0.05) | 2,402 (0.02) | 228 (0.002) | 4,785 (0.06) | 2,385 (0.03) | 228 (0.003) |
| El Salvador | 2,980 (0.03) | 430 (0.004) | 14 (0.0001) | 2,199 (0.03) | 297 (0.003) | 14 (0.0002) |
| Total | 9,238,072 (100) | 5,721,975 (61.93) | 1,644,964 (17.81) | 7,787,759 (100) | 4,726,649 (60.69) | 1,381,237 (17.74) |
The current PAs system in the Neotropics represents ~20 % of species’ potential distribution (Figure 2c). Areas inside PAs showed significantly higher suitability values (0.351 ± 0.276; KS, P < 0.001) than areas outside them (0.319 ± 0.238). The highest values were obtained in the Amazon areas (including Bolivia, Peru, Ecuador, Colombia, Venezuela, and Brazil; Figure 2), followed by the Guiana shield and the coast of the Atlantic forest. The index of suitable value multiplied by its distance to nearest occurrence and river identified gaps (index > 0.5) within the distribution that need attention during future surveys, such as the frontier between Venezuela and Guyana (mainly in the Guiana Highlands), the Amazonian region (including Colombia and the northwestern Brazil), and central-eastern Brazil (Figure 3a).
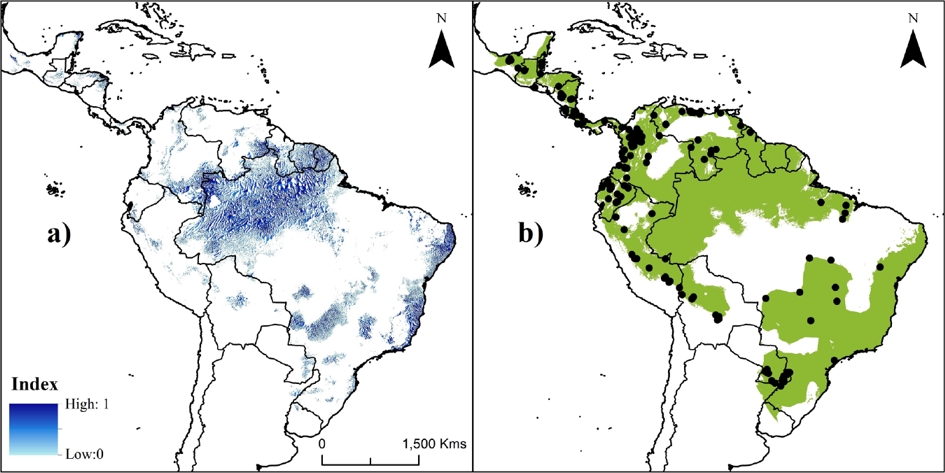
Figure 3 Maps showing priority areas for future studies (a) and the current proposed Neotropical distribution for water opossum, Chironectes minimus (b). Color palette in A corresponds to areas defined as priorities (from zero [light blue] to 1 [dark blue]) for future ecological studies and surveys for water opossum based on suitable value multiplied by its distance to nearest occurrence and water source (i. e., rivers). Black points in B represent the unique historical records (n = 165) of species.
Discussion
Potential distribution range of water opossum and habitat loss effects. Our results confirm that the climate variables used in this study (Table 1) can be employed to model the potential distribution of terrestrial species associated with aquatic environments, as previously demonstrated for the otters Lontra longicaudis and Pteronura brasiliensis (Cianfrani et al. 2011; Rheingantz et al. 2014). Mean precipitation of the driest quarter and the warmest quarter were the most important variables for the water opossum’s distribution in the Neotropics (Table 1), as was found for the Neotropical otter (Rheingantz et al. 2014). Altitude was another important variable which represents a gradient correlating directly with factors such as micro-climate or edaphic conditions (Mota-Vargas et al. 2013; Kübler et al. 2016). Although water opossum occurred between zero to ~3,000 m (including the Andes region), most of the occurrences were between zero to 500 m (n = 81) and zero to 2,000 m (n = 157; Appendix 1). This spatial distribution of species’ occurrences suggests that the species has an altitudinal limit (due to climatic gradients by elevation) possibly associated with their physiological requirements. This last idea agrees with studies for the Neotropical otter, which is described as abundant at medium elevations (Lariviére 1999; Rheingantz et al. 2014).
It is important to observe that suitability model predicted for C. minimus was severely reduced due to habitat loss (~36 to 43 %); even inside of PAs (Tables 2 and 3). The habitat loss is associated with areas highly threatened by human activities (e. g., expansion of cattle ranching and urban settlements), which remove vegetation cover thereby reducing water opossum’s habitat (Prieto-Torres et al. 2008; 2011; Galliez et al. 2009). Similarly, previous studies report that the expansion of the agricultural frontier is a critical factor affecting biodiversity in the Neotropics (Shukla et al. 1990; Lees and Peres 2006; Bressiani and Graipel 2008; Ribeiro et al. 2009; Ortega-Andrade et al. 2015; Prieto-Torres et al. 2016). These conditions push the species to the edge of its distribution and increase fragmentation of predicted suitable areas, which could promote decreasing trends in populations (Ardente et al. 2013; Palmeirim et al. 2014; Fernandez et al. 2015). Thus, future conservation efforts should concentrate on reducing habitat loss and restoring identified natural habitats, especially considering the restricted home range and unknown population size of the water opossum (Galliez et al. 2009; IUCN 2015).
Protected areas and gaps in areas for future studies. We demonstrated that PAs included areas with high habitat suitability values for C. minimus, which could protect it in the medium and long-term. Furthermore, our analysis supports the idea that SDMs can be used to evaluate whether PAs are really conserving species within them. Such studies allow us to identify potential areas of conservation priority for the species to achieve more realistic conservation goals in their present and future distributions (e. g., Hannah et al. 2005, 2007; Dudley and Parish 2006; Lessmann et al. 2014).
The PAs system is especially important for the water opossum in the Amazon region, due to the low rate of deforestation of the remaining forest (Numata and Cochrane 2012). The persistence of PAs in this region will play a role in preventing environmental degradation in the central and south portion of the C. minimus. Meanwhile, populations along the Mesoamerican region (from Mexico to Panama), the western Andes (Ecuador, Colombia and Venezuela), and southeastern Brazil are more vulnerable to the effects of forest loss due to fewer PAs (Figure 2b-c). However, it is important to conserve not only PAs but also surrounding areas through forest restoration and sustainable development programs which include local people (Laurance et al. 2012; Rheingantz et al. 2014; Prieto-Torres et al. 2016). Additionally, studies under future climate change scenarios are needed to consider the role of the PAs system in protecting the species’ habitat (e. g., Hannah et al. 2005, 2007).
We suggest that future studies (e. g., inventories, population monitoring, abundance patterns, and habitat evaluations) need to be focused on the Guiana Highlands, the Amazonian region (including Colombia and northwestern Brazil), and central-eastern Brazil (Figure 3a). Working in unexplored areas frequently provides new information on a species in the form of expansion of known distribution ranges and new records of unidentified specimens (Soberón and Peterson 2005; Mota-Vargas and Rojas-Soto 2012; Tôrres et al. 2012; Ortega-Andrade et al. 2013; Rheingantz et al. 2014). Thus, our results aid in identifying unexplored areas where future survey efforts should be focused in order to accelerate the discovery of new populations of water opossum.
Implications for C. minimus’ conservation. Our results showed areas absent from the IUCN’s distribution map, indicating that this needs to be updated. Thus, we proposed a new tentative extent of the water opossum distribution (Figure 3b) which integrated the information obtained in the SDMs, the IUCN historical range, and the newly reported localities. This proposal includes new distribution areas for Mexico, Venezuela, Suriname, Guyana, Ecuador, Peru, Bolivia, and Argentina-Brazil; and at the same time reduces or eliminates areas in northern Brazil and the savanna in Colombia and Venezuela.
Clearly, the limited knowledge about the habitat requirements, distribution range, and information obtained directly from field activities, could explain why the water opossum is currently listed as Least Concern. At the continental level, there are mammals which have been reassigned because threat categories have been based more on anecdotal criteria than on field surveys and population assessments (e. g., Rheingantz and Trinca 2015). Apart from the problems associated with the lack of data for its categorization (Cuarón et al. 2008), our results in combination with the time elapsed since the first assignment justifies the need for a reassessment of the category, such as was done for L. longicaudis, whose threat category was up-listed from “Least Concern” to “Near Threatened” (Rheingantz and Trinca 2015).
On the other hand, it is important to note that our models showed that there is a disjunction in the distribution of water opossum, observed in the population of southeastern of Brazil (C. m. paraguanensis [Marshall 1978; Damasceno and Astúa 2016]). This disjunct distribution could represent ecological niche differences among subspecies, which could simultaneously affect the performance of our models (see Rojas-Soto et al. 2008; 2009; Mota-Vargas and Rojas-Soto 2016). It is reasonable to suggest that there are climatic and geographic factors acting (or that acted) as geographic barriers that contribute to the isolation of some populations (see Damasceno and Astúa 2016). Similar cases were documented for wide-ranging Didelphidae species: the genus Didelphis, the Black-eared opossums (D. marsupialis; Cerqueira 1985) and White-eared opossums (D. albiventris; Cerqueira and Lemos 2000), and the Lutrine Opossum, Lutreolina crassicaudata (Martínez-Lanfranco et al. 2014). From this perspective, our study suggests that the current taxonomic status of these populations needs to be adequately assessed using tools that could reveal their distinctiveness (Damasceno and Astúa 2016). Independently of the current taxonomic classification, a possible loss of one of these disjunct groups would be irreversible.
Although we only examined the environmental distribution of C. minimus, our results forecast a rapid decline in the potential distribution, principally attributed to a decrease in occupancy in areas affected by habitat loss and fragmentation (e. g. Prieto-Torres et al. 2011; Ardente et al. 2013; IUCN 2015; Palmeirim et al. 2014; Fernandez et al. 2015). Modifications to the physicochemical characteristics (e. g., water conditions) of the habitat due to the aforementioned processes can considerably affect water opossum populations and reduce local diversity, as found for other aquatic mammals (e.g., Bowyer et al. 1995; Rheingantz et al. 2014). Thus, considering physicochemical water conditions, the habitat structures to persist, and the habitat requirements to establish a viable population, will be crucial for the conservation of C. minimus and the preservation of river ecosystems as a whole.











 nueva página del texto (beta)
nueva página del texto (beta)


