Introduction
The jaguar (Panthera onca) and the puma (Puma concolor) are the largest felids in the Neotropics since the Late Pleistocene, and these species constitute the guild of large apex predators in terrestrial ecosystems there. Jaguars and pumas are sympatric throughout the entire range of the jaguar, and pumas are smaller in areas where they are sympatric with the jaguar, but they have a larger body size outside the areas of jaguar distribution (Iriarte et al. 1990; Sunquist and Sunquist 2009). This pattern has been explained as a type of character displacement facilitating avoidance of competition (Iriarte et al. 1990). Character displacement is defined as the process in which differences in one or more morphological characters among similar species with overlapping distributions are accentuated as a result of inter-specific competition in regions where the species occur together; these differences are minimized or lost where the distributions of the two species do not overlap (Brown and Wilson 1956; Dayan et al. 1990; Jones 1997; Pfennig and Pfennig 2009).
Although jaguars are larger than pumas where they are sympatric, there is overlap in their size and the size of prey they can hunt effectively; for this reason, several authors have considered them as potentially competing species (Taber et al. 1997; Nuñez et al. 2000; Scognamillo et al. 2003; Novack et al. 2005; Azevedo 2008; Rosas-Rosas et al. 2008; Foster et al. 2010). Several studies have examined the interactions between these species in areas of sympatry. It appears that coexistence between jaguars and pumas is facilitated by using different habitats (Scognamillo et al. 2003), active avoidance of the same sites (Harmsen et al. 2009; Sollmann et al. 2012; de la Torre et al. 2017), temporal segregation (Romero-Muñoz et al. 2010), or by differential use of prey (Emmons 1987; Jorgeson and Redford 1993; Aranda and Sánchez-Cordero 1996; Taber et al. 1997; Nuñez et al. 2000; Scognamillo et al. 2003; Novack et al. 2005). However, few studies have analyzed the differences in body sizes and other morphological characters of these coexisting species (Kiltie 1984; Morales and Giannini 2010), especially from data obtained directly from animals of the same study area.
We examined and compared body and cranio-dental measures of sympatric jaguars and pumas in a tropical rainforest of Mexico. Because pumas are smaller in areas where they are sympatric with the jaguar (Iriarte et al. 1990), we predicted that morphological characteristics of these species would differ in southern Mexico. Given that felid species are strongly sexually dimorphic and each sex of a guild of felids can be considered as morphologically distinct (Dayan et al. 1990; Jones 1997), we also predicted that morphological characteristics would differ within each species between males and females.
Materials and methods
Data on body sizes of jaguars and pumas were obtained from the Greater Lacandona Ecosystem (GLE) in southeastern Mexico (Medellín 1994; de la Torre and Medellín 2011). Jaguars and pumas were captured in foot snares and fitted with GPS radio-collars (Frank et al. 2003; de la Torre et al. 2017). At the site of each snare trap, we also placed a VHF radio transmitter to monitor if traps were triggered (Halstead et al. 1995). Traps were checked every four hours throughout the night and, depending on weather conditions, several times during the day to respond immediately to any capture. All capture and handling protocols followed the IACUC Guidelines of the American Society of Mammalogists (Sikes et al. 2011). Permission to conduct the field captures was granted by the General Office of Wildlife-SEMARNAT (2010-No.11347). After capture, we immobilized jaguars using a dose of 0.08 mg/kg of medetomidine combined with 5 mg/kg of ketamine, and pumas using a dose 0.08 mg/kg of medetomidine combined with 6 mg/kg of ketamine. Immobilization dosage was administrated using a dart fired from a CO2 pistol or rifle. While the animal was immobilized, we examined individual body condition and determined sex. We estimated age based on coat color, tooth wear (Stander 1997), and gum-line recession (Laundré et al. 2000). Body mass and linear measurements were recorded. Body mass (weight) was recorded using a portable scale. All captured animals were released after their examination.
We contrasted the body mass ratio between species and sexes. For this we used the formula, (M-F)/[(M+F)/2]; where M is the average body mass of the males, and F is the average body mass of the females. This formula provides the difference in mass relative to the average mass of the sexes. In the case of the comparisons between different species of the same sex, we treat the species with the greater body mass (jaguars) as males (M), and the species with lesser body mass (pumas) as females (F).
We analyzed body mass, body length (head + body), tail length, head length, and shoulder height. We used the cube root of the body mass because the other measures are linear, while body mass refers to volume. Additionally, we used the length of the superior canines, distance between superior canines, distance between inferior canines, diameter of superior canines, and diameter of inferior canines. All measurements were log-transformed to improve normality, and all transformed variables were included in the analyses. We conducted a Principal Component Analysis (PCA) to evaluate and visualize variability in size between species and sexes, and to characterize groups of individuals according to these morphological variables. We performed the PCA using the function “prcomp” available in STATS package of R 3. 1. 1 (R Core Team 2016).
Because our sample size was small, we compared morphological variables using a one-way analysis of variance (ANOVA) to assess the significant differences between species and sexes. We used the species and sexes arranged in four different groups (male jaguar, female jaguar, male puma and female puma) as explicative variables and the characters measured as response variables. If the ANOVA result was significant, we additionally implemented a pairwise comparison to determine significant differences between the groups for each character measured. We used a Bonferroni adjustment with the aim to control the Type I error across the pairwise tests. We assumed a significant α level of 0.05 for all tests. We performed the ANOVAs and pairwise comparisons using the functions “lm” and “pairwise.t.test” respectively available in STATS package of R 3. 1. 1 (R Core Team 2016).
Results
Five jaguars (2 males and 3 females) and 11 pumas (8 males and 3 females ) were captured from November 2011 through April 2013 (Table 1). All individuals captured were classified as adults except for two male pumas which were juveniles. Using the average of body mass, male jaguar versus female jaguar ratio was 0.42, and in male puma versus female puma was 0.37. In the other hand, male jaguars were 0.50 times heavier than male pumas and female jaguars were 0.46 times heavier than female pumas. However, male puma versus female jaguar body mass ratio was –0.09, indicating that female jaguars are slightly heavier than male pumas in the southern Mexico.
Table 1 Mean and standard errors of body mass, body measurements and craniodental measurements of jaguars and pumas of southern Mexico. Body mass is in kg, all other measurements are in centimetres.
| Panthera onca | Puma concolor | |||
|---|---|---|---|---|
| Male (n = 2) | Female (n = 3) | Male (n = 8) | Female (n = 3) | |
| Body mass | 52.50 ± 3.53 | 34.33 ± 1.52 | 31.25 ± 5.23 | 21.33 ± 1.15 |
| Body length | 131.50 ± 3.50 | 118.33 ± 4.04 | 122.87 ± 5.89 | 108.66 ± 3.05 |
| Tail length | 53.00 ± 5.65 | 51.33 ± 4.16 | 63.00 ± 5.26 | 62.00 ± 3.00 |
| Head length | 32.00 ± 0.00 | 27.50 ± 0.86 | 25.75 ± 1.83 | 23.33 ± 0.57 |
| Shoulder height | 58.50 ± 0.70 | 51.00 ± 1.00 | 50.50 ± 2.67 | 46.00 ± 1.00 |
| Superior canine length | 3.45 ± 0.10 | 3.36 ± 0.17 | 2.49 ± 0.15 | 2.20 ± 0.08 |
| Distance between superior canines | 4.11 ± 0.20 | 3.65 ± 0.20 | 3.09 ± 0.14 | 2.83 ± 0.15 |
| Distance between inferior canines | 2.49 ± 0.01 | 2.43 ±0.18 | 1.92 ± 0.16 | 1.90 ± 0.14 |
| Superior canine diameter | 1.78 ± 0.04 | 1.49 ± 0.05 | 1.19 ± 0.07 | 1.08 ± 0.15 |
| Inferior canine diameter | 1.55 ± 0.09 | 1.43 ± 0.17 | 1.06 ± 0.09 | 0.79 ± 0.09 |
The PCA analysis grouped the 16 measured individuals into four groups: male jaguars, female jaguars, male pumas, and female pumas, although with some overlap of the 95 % confidence intervals for male and female pumas (Figure 1). The first axis explained 73.5 % of the variance and grouped the male and female jaguars on the negative side of this axis by their body mass, head length, and superior canine length and diameter (Table 2). Meanwhile, male and female pumas remained on the positive side of the first axis, which means that jaguars are larger in these characteristics than pumas in southern Mexico. The second axis explained 13.1 % of the variance and grouped the individuals basically by their head + body length and shoulder height (with positive character loadings), and distance between inferior canines (negative loading). This axis represented size associated with body length and height, after the general size effect is removed. The positive side of the second axis included male jaguars and male pumas, and the negative side grouped the female jaguars and female pumas, which demonstrated that males of both species had relatively larger body lengths and heights than conspecific females, whereas females had relatively greater breadth between lower canines (Table 2, Figure 1).
Table 2 Loadings for all variables for the first three principal components, and the variance explained, the cumulative variance explained and the standard deviation for the first three principal components.
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Body mass | -0.3409 | 0.2616 | -0.1726 |
| Head + body length | -0.2550 | 0.5368 | -0.2930 |
| Tail length | 0.2296 | 0.5115 | 0.6202 |
| Head length | -0.3400 | 0.1987 | -0.1753 |
| Shoulder height | -0.3270 | 0.3665 | 0.0111 |
| Superior canine length | -0.3491 | -0.1914 | 0.0431 |
| Distance between superior canines | -0.3395 | -0.1358 | 0.3515 |
| Distance between inferior canines | -0.3013 | -0.3548 | -0.1589 |
| Superior canine diameter | -0.3357 | -0.1264 | 0.2457 |
| Inferior canine diameter | -0.3208 | -0.1052 | 0.5078 |
| Proportion of variance explained | 0.7349 | 0.1308 | 0.0525 |
| Cumulative proportion | 0.7349 | 0.8658 | 0.9183 |
| Standard deviation | 2.7110 | 1.1439 | 0.7246 |
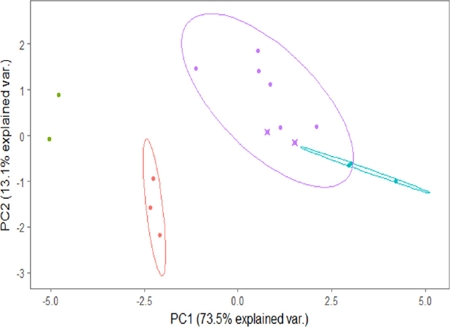
Figure 1 Principal Component Analysis of body and cranio-dental measurements. The first axis (PC1) accounts for 73.5 % of the total variation and, the second axis for 13.1 % of the total variation. See Table 2 for character loadings on these two components. Ellipses represent the 95 % confidence ellipses for each group (female jaguars, male pumas and female pumas). Green points represent male jaguars; red points female jaguars; purple points male pumas and turquoise points female pumas. The two juvenile pumas evaluated in this study include an “x” in the circle.
With the ANOVA we detected significant differences between the species and sexes in all characters measured (for all characters, d. f. = 3, n = 16): body mass, F = 22.64, P < 0.0001; body length, F = 9.40, P < 0.01; tail length, F = 5.75, P < 0.05; head length, F = 15.26, P < 0.001; shoulder height, F = 13.83, P < 0.001; superior canine length, F = 44.43, P < 0.0001; distance between superior canines, F = 63.10, P < 0.0001; distance between inferior canines, F = 14.96, P < 0.001; diameter of superior canines, F = 33.62, P < 0.0001; and diameter of inferior canines, F = 19.97, P < 0.0001.
The pairwise comparisons revealed that jaguars were heavier than pumas, and that males of both species were heavier than females of the same species (Table 3). However, the pairwise comparison showed that body mass between female jaguars and male pumas were not significant different (Table 3). Furthermore, comparing all body measurements between female jaguars and male pumas, we detected significant differences in tail length only; male pumas had longer tails. These results indicate that female jaguars and male pumas were similar in body mass and size in southern Mexico (Table 1, Table 3).
Table 3 Results (P-values) of the pairwise comparisons using the Bonferroni adjustment between jaguar and puma morphometric measurements in the Greater Lacandona Ecosystem, southern Mexico (body mass = BM; body length = BL; tail length = TL; head length = HL; shoulder height = SH; superior canine length = SCL; distance between superior canines = DBSC; distance between inferior canines = DBIC; superior canine diameters = SCD; inferior canine diameters = ICD).
| Pair contrast | BM | BL | TL | HL | SH | SCL | DBSC | DBIC | SCD | ICD |
|---|---|---|---|---|---|---|---|---|---|---|
| Female jaguar vs. female puma | 0.0153 | 0.2236 | 0.1140 | 0.0268 | 0.0840 | < 0.0001 | < 0.0001 | 0.0036 | 0.0007 | 0.0003 |
| Male jaguar vs. female jaguar | 0.0029 | 0.0873 | 1.0000 | 0.0333 | 0.0137 | 1.0000 | 0.02444 | 1.0000 | 0.0233 | 1.0000 |
| Female jaguar vs. male puma | 1.0000 | 1.0000 | 0.0230 | 0.6152 | 1.0000 | < 0.0001 | 0.0004 | 0.0011 | 0.0007 | 0.0073 |
| Male jaguar vs. female puma | < 0.0001 | 0.0020 | 0.3800 | 0.0001 | 0.0002 | < 0.0001 | < 0.0001 | 0.0099 | < 0.0001 | 0.0002 |
| Male puma vs. female puma | 0.0268 | 0.0081 | 1.0000 | 0.1866 | 0.0532 | 0.087 | 0.1227 | 1.000 | 0.6717 | 0.0543 |
| Male jaguar vs. male puma | 0.0002 | 0.3114 | 0.1333 | 0.0009 | 0.0028 | < 0.0001 | < 0.0001 | 0.0048 | < 0.0001 | 0.0027 |
However, we found a different pattern in the cranio-dental characters. Jaguars had longer canines, greater distances between both superior and inferior canines, and a greater diameter in superior and inferior canines, than did pumas (Table 3). These differences between female jaguars and male pumas were significant for all cranio-dental characters (Table 3). Thus, although female jaguar and male pumas had similar body mass and size in southern Mexico, the size and robustness of canines and the mouth size were larger in female jaguars than in male pumas (Table 1, Table 3).
Discussion
Although sample size was small, we found significant differences in cranio-dental sizes between jaguars and pumas in southern Mexico. This finding suggests that character displacement between jaguars and pumas might be expressed not only by their body size, but also by their skull size, the length and robustness of canines, and breadth of the dental arcade. Caveats of this conclusion include that our sample size is small and that we did not include information of pumas from areas where this species is not sympatric with the jaguars. However, a larger cranio-dental size could confer an advantage to jaguars for hunting larger prey and for exploiting species that pumas are not able to use, although some overlap in body size between these two species may occur where they are sympatric (Emmons 1987; Taber et al. 1997).
Felid species show different morphological adaptations correlated with the size of prey taken (Meachen-Samuels and Van Valkenburgh 2009a, b; Sicuro and Oliveira 2011). Species that specialize in larger prey are distinguished by having a larger skull, more robust canines, a wider mouth, and a larger opening angle of the mouth. These characteristics are advantageous for dominating and killing large prey. On the other hand, felid species that specialize in taking small prey have smaller canines, a narrower mouth, a slightly longer jaw, and a greater bite force than the larger species relative to their body mass, and these features enhance their ability to capture small but agile prey (Christiansen 2007, 2008; Meachen-Samuels and Van Valkenburgh 2009a, b; Slater and Van Valkenburgh 2009; Sicuro and Oliveira 2011).
Generalist species, such as pumas, exhibit characteristics that are intermediate between these two groups, which indicates that they are adapted for taking prey of both sizes (Christiansen 2007; Meachen-Samuels and Van Valkenburgh 2009a, b). Additional evidence comes from the spectrum of prey taken in areas of sympatry. Pumas hunt a wider spectrum of prey than jaguars in areas of co-occurrence, and they often take smaller prey relative to jaguars (Rabinowitz and Nottingham 1986; Emmons 1987; Nuñez et al. 2000; Scognamillo et al. 2003; Novack et al. 2005).
Moreover, larger and more robust canines could enable jaguars to exploit other type of prey species that pumas are not able to use, or that they use with lower frequency than jaguars. For instance, it has been documented that due to the strength of their bite, jaguars can feed on vertebrates with hard skins or very resistant shells, such as crocodiles, turtles or armadillos (Rabinowitz and Nottingham 1986; Emmons 1989; Nuñez et al. 2000; Da Silveira et al. 2010; Arroyo-Arce and Salom-Pérez 2015; Guilder et al. 2015), and the occurrence of this kind of species in jaguar diet is greater than in pumas (Emmons 1987; Taber et al. 1997; Nuñez et al. 2000; Scognamillo et al. 2003; Foster et al. 2010).
It is important to consider that two of the male pumas evaluated in this study were classified as juveniles by their body and dentition condition. This probably could affect our results because the group that presented more variability in the body characters measured was the male pumas (Table 1). Nevertheless, cranio-dental characters in male pumas exhibited variability similar to that of the other three groups (male jaguars, female jaguars, and female pumas). This suggests that the effect of the juvenile individuals included in our analysis was reflected only in the body size variables.
By comparing the ratios of body mass between jaguars and pumas in sympatry, which were obtained from field projects carried throughout American continent, we observed that ratios of body mass between these species varied throughout their sympatric range (Table 4). Mean ratio in body mass between sexes of the same species ranged from 0.30 to 0.58 in jaguars and from 0.37 to 0.55 in pumas; between the same sex in jaguars and pumas the mean ratio in body mass ranged from 0.21 to 0.57 in males, and from 0.46 to 0.79 in females (Table 4). In general, male jaguars were larger than male pumas and female jaguars were larger than female pumas.
Table 4 Mean ratios of body mass between sexes (males M, females F) and species throughout the sympatric distribution of jaguars (J) and pumas (P).
| Study area | Latitude | Sample size | FJ vs MJ | FP vs MP | FJ vs FP | MJ vs MP | FJ vs MP | MJ vs FP | |
|---|---|---|---|---|---|---|---|---|---|
| Jaguars | Pumas | ||||||||
| Jalisco, Mexicoa | 19° N | 2 ♂; 4 ♀ | 3 ♂ | 0.51 | 0.21 | 0.31 | |||
| Lacandona Forest, Mexicob | 16° N | 2 ♂; 3 ♀ | 8 ♂; 2 ♀ | 0.42 | 0.37 | 0.46 | 0.50 | -0.09 | 0.84 |
| Los Llanos, Venezuelac | 8° N | 3 ♂; 2 ♀ | 2 ♂; 4 ♀ | 0.58 | 0.67 | 0.62 | 0.53 | 0.05 | 1.10 |
| Emmas, Brazild | - 18° S | 2 ♂ | 3 ♂; 4 ♀ | 0.54 | 0.48 | 0.96 | |||
| El Chaco, Paraguaye | - 21° S | 13 ♂; 9 ♀ | 6 ♂; 2 ♀ | 0.30 | 0.55 | 0.79 | 0.57 | -0.28 | 1.03 |
| Mean | 0.45 | 0.53 | 0.62 | 0.46 | 0.00 | 0.98 | |||
| Standard deviation | 0.12 | 0.12 | 0.17 | 0.14 | 0.24 | 0.11 | |||
a Nuñez, 2006, bThis study.cScognamillo et al., 2003, dSilveira, 2004, eMcBride, 2009
However, apparently female jaguars can have a smaller, similar or greater body mass than male pumas within their sympatric range (Table 4). For instance, female jaguars are smaller than male pumas in Jalisco, Mexico, have similar size to male pumas in southern Mexico and Los Llanos, Venezuela, and are larger than male pumas in the Chaco of Paraguay. The sample size of the studies analyzed was small, but this pattern probably is due because jaguars are smaller at the northern limit of their distribution range and their body mass increase towards South America. The variation in jaguar size seems is related with the availability of largest prey. Largest jaguars occur in the open floodplain habitats where they take largest prey, while smallest jaguars occur in forest habitats where they take smallest prey (Hoogesteijn and Mondlofi 1996; Sunquist and Sunquist 2009). This implies that female jaguars and male pumas could have similar body mass and size in much of their sympatric distribution, and that their cranio-dental differences have probably facilitated the resource partitioning and coexistence of these sympatric species that have substantially overlapping ecological requirements.











 nueva página del texto (beta)
nueva página del texto (beta)


