Introduction
Mountain viscachas of the genus Lagidium Meyen 1833 (Rodentia, Chinchillidae) are medium-to-large hystricomorph rodents (1.5 to 3 kg) that live in rocky outcrops from Ecuador to southern Argentina and Chile (Spotorno and Patton 2015). The taxonomic history of this genus, similar to other Neotropical mammals, can be divided into three main stages. During the first period, that spans between the late 18th century and the early 20th century, more than 20 nominal forms were described, mostly based on one or two specimens from their respective type localities (e. g., Thomas 1907). At the second stage, under the paradigm of the biological species concept, most of these names were regarded as synonyms, depending upon the author (some of them recognized as subspecies), of three to four more widely distributed species (e. g., Cabrera 1961). Finally, the use of molecular markers in recent years suggested that this scenario is not representative of the real taxonomic diversity within the genus (Spotorno et al. 2004, Ledesma et al. 2009).
Hayman (in Ellerman 1940:230-231), based on the specimens housed at the British Museum, was the first reviewer of the genus Lagidium. This author recognizes four species, from north to south: L. peruanum Meyen 1833, L. viscacia (Molina 1782), L. boxiThomas 1921, and L. wolfsohni (Thomas 1907). Hayman (in Ellerman 1940) used size, presence of dorsal stripes and coloration pattern as the main diagnostic features for delimiting the different species. Subsequent authors subsumed boxi (e. g.,Cabrera 1961) and peruanum (e. g., Spotorno and Patton 2015; but see Ledesma et al. 2009) under L. viscacia. Overall, the profusion of names within this genus was reflected by the recognition of multiple subspecies, especially within viscacia (e. g., Cabrera 1961; Crespo 1963; Mann 1978). More recently, Spotorno et al. (2004) and Ledesma et al. (2009), combining molecular and morphometric evidences in the context of the description of a new species from Ecuador (L. ahuacaense), recognized several molecular lineages within Lagidium that partially coincide with the groups recovered in their multivariate analysis of metrical data. In addition, Ledesma et al. 2009 found that L. viscacia, as currently conceived, was not resolved as a monophyletic group. Recently, Spotorno and Patton (2015), in a conservative approach, recognized only three species (L. ahuacaense, L. viscacia, and L. wolffsohni) and discussed extensively the taxonomic history of the genus (see Table 1 for a synthesis of the main taxonomic hypothesis within Lagidium). Taxonomic uncertainties within Lagidium are related to the deficient definition of some taxa, the apparently limited morphological differentiation between species, and the poor representation of specimens in biological collections (Spotorno and Patton 2015).
Table 1 Main taxonomic hypothesis for the species of the genus Lagidium (Rodentia, Chinchillidae), including the arrangement of subspecies proposed by different authors.

In this work we studied, from a qualitative and quantitative approach, Argentinean and Bolivian populations referred to L. viscacia, in order to better understand their geographic morphological variation pattern. Based on our results, we consider that taxonomic changes are needed.
Materials and Methods
One hundred and fifty specimens referred to as Lagidium viscacia from Argentina and western Bolivia were examined in this study (see Appendix 1). For comparative purposes, we also included six specimens of L. peruanum from northern Chile. Sixteen craniodental variables were measured in adult specimens (n = 55) in order to quantitatively describe the size and shape of the major skull structures, as follows (Figure 1): skull length (SL), condylo-incisive length (CIL), zygomatic breadth (ZB), braincase breadth (BB), palatilar length (PalL), incisive foramina length (IFL), incisive foramina width (IFW), diastema length (DL), maxillary toothrow length (alveolar) (TRL), palatal width at M3 (PWM3), breadth across paraoccipital process (BPP), nasal length (NL), nasal width (NW), interorbital breadth (IB), frontal length (FL), and bullar length (BuL). All measurements were obtained with digital calipers to the nearest 0.05 mm, and were log-transformed before the multivariate statistical analyses.
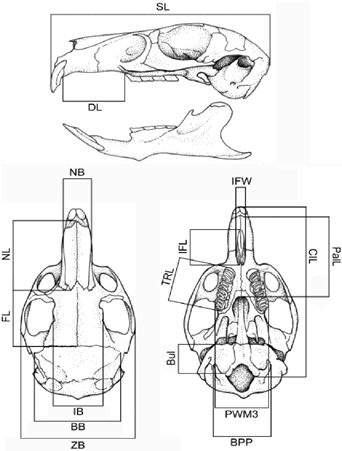
Figure 1 Measurements used in the multivariate analysis. For measurement abbreviations: see the section Materials and Methods.
Geographic trends and the degree of differentiation between samples were examined by multivariate statistical procedures, including principal component analysis (PCA) and canonical variate discriminant analysis (DA). Small samples from different localities were grouped following the geographic proximity criterion (e. g., Musser 1968) and the similarity between the geomorphological characteristics of the localities sampled (e. g., mountain slopes, relief; see Chiquito et al. 2014). Samples with only one individual were not included in DA. Samples were labeled in the figures and tables using the following abbreviations: Argentina: CH = Chubut Province, JU = Jujuy Province, LR = La Rioja Province, ME = Mendoza Province, NQ = Neuquén Province, RN = western Río Negro Province, SA = Salta Province, SJ = San Juan Province, SO = Somuncurá, TU = Tucumán Province. Bolivia: BO = western Bolivia. Chile: PE = Northern Chile (Figure 2C). The sample from RN includes two topotypes of L. boxi Thomas 1921, and the sample from SO includes the type series of L. v. somuncurensisCrespo 1963. Previous studies on Lagidium have shown that differences between sexes are not significant (cf. Pearson 1948); consequently, we pooled males and females in the multivariate analyses.
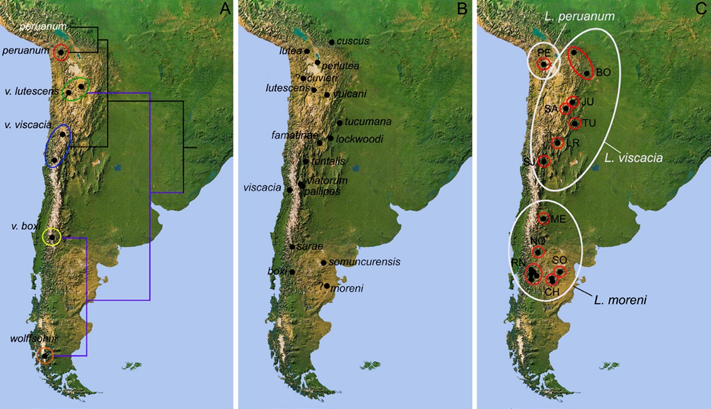
Figure 2 Map of southern South America depicting: A) A simplified tree of the phylogenetic hypothesis for Lagidium based on cytb sequences presented by Ledesma et al. (2009). B) Type localities of the nominal forms included by Spotorno and Patton (2015) within the synonymy of Lagidium viscacia (e. g., Cabrera 1961; Mann 1978; Anderson 1997; the nominal forms L. crassidens Philippi 1896 and L. crinigerum Phillipi 1896, included by Osgood 1943 under the synonymy of L. v. viscacia, were not mapped due to uncertainties about their type localities. C) Geographic samples defined in this work (see Materials and Methods for abbreviations). White ellipses illustrate the taxonomic hypothesis proposed in this work.
The variation in cranial and external qualitative anatomical characters was also documented. The anatomical terminology used to describe skull structures follows Cherem and Ferigolo (2012). Pelage coloration was assessed by side-by-side comparisons of specimens.
Results
The principal component analysis revealed two major morphometric groups spanning along the 1st and 2nd principal components, which accounted for ~70 % of the variance (Figure 3, Table 2). All craniometric characters were positively correlated with PC1, indicating size variation as the main source of differentiation between samples (Figure 3, Table 2). The first group was composed of specimens from southern Argentina (i. e., CH, NQ, RN and SO), while the second encompassed animals from northwestern Argentina (i. e., JU, LR, SA, SJ, TU) and western Bolivia (BO) plus L. peruanum from northern Chile (PE). This latter sample (PE) appeared as the smallest in cranial size within this second group. Overall, the overlap in multivariate space among geographic samples within the first and second groups was moderate to high (Figure 3).

Figure 3 Specimen scores of adult individuals of Lagidium (n = 55) for Principal Components 1 and 2 (left), and for Canonical Variates 1 and 2 extracted from an eight-group discriminant function analysis (right). See Materials and Methods for the explanation of the abbreviations.
Table 2 Specimen scores of adult individuals of Lagidium (n = 55) for Principal Components 1 and 2, and for Canonical Variates 1 and 2 extracted from an eight-group discriminant function analysis. See materials and methods for the explanation of the abbreviations.

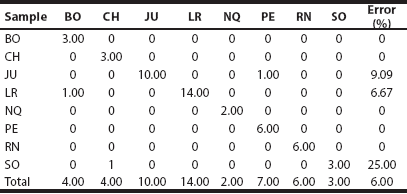
The discriminant analysis correctly allocated almost all specimens in their respective geographic sample (Table 3). The 1st and 2nd canonical variates accounted for ~80 % of the variance. Specimens from southern Argentina (i. e., CH, NQ, RN and SO) differed from the other samples by having overall larger and more robust crania, with markedly larger characters related to BB, BPP, PalL, and NW (Figure 3, Table 2 and 3).
Table 3 Classification matrix of geographic samples of Lagidium determined by the eight-group discriminant function analysis (see also Figure 3 and Table 2). See Materials and Methods for the explanation of the abbreviations.
Among the Argentinean samples, the two main groups identified through PCA can be diagnosed based on the distribution of qualitative characters. The external coloration was relatively variable among samples, a fact previously noted by Pearson (1948). However, there is a clear predominance of grayish-colored specimens, with well-marked and usually broad dorsal stripes among southern (CH, RN, SO; Figure 4A) and west-central (ME) Argentinean samples and yellowishgray individuals (more or less suffused with orange), with diffuse to well-marked, usually narrow, dorsal stripes among the populations from northwestern Argentina (JU, LR, SA, TU; Table 4; Figure 4B). Individuals from NQ were metrically nested within samples from southern Argentina, although their external coloration was mostly yellowish-gray. A main difference between both groups was the shape of nasals, which were relatively large with a conspicuous widening in the distal half in specimens from southern Argentina (i. e., CH, NQ, RN and SO), and narrow with nearly straight and parallel borders in the remaining samples (i. e., JU, LR, SA, SJ, TU; Figure 5). In addition, the dorsal root of the zygomatic process of the maxilla was narrow in the first group and broad in the second (Figure 5).
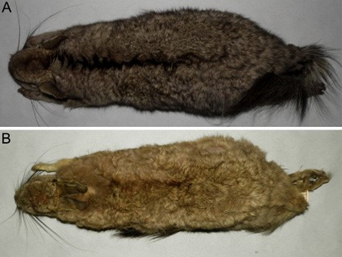
Figure 4 External view of the dorsal coloration of different populations of Lagidium: A) southern Argentina (MACN 14313; Pilcaniyeu, Río Negro; here referred as L. moreni). B) north-western Argentina (MACN 34.258; Sierra de Velazco, La Rioja; here referred as L. viscacia). Not in scale.
Table 4 Variation on the occurrence of different types of dorsal coloration and development of the dorsal stripe among geographic samples of Lagidium. See materials and methods for the explanation of the abbreviations.

1Two specimens from RN have a nearly uniform orange coloration, darker at the midline (MACN 36.135, 36.136; skulls unavailable); one specimen from SJ has a light gray dorsum frosted with white, and a light orange venter (MACN 18829).
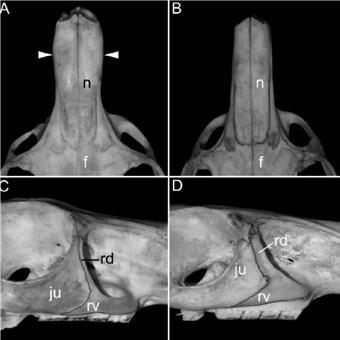
Figure 5 Anatomic details of the skulls of Lagidium moreni (A, C) and L. viscacia (B, D): A, C) MACN 13933 (NQ), note the nasals [n] anteriorly expanded and the narrow dorsal root of the zygomatic process of the maxilla [rd]; B, D) MACN 18829 (JU), presenting nasals [n] not expanded anteriorly and a broad dorsal root of the zygomatic process of the maxilla [rd]. Other abbreviations: f = frontal; ju = jugal; rv = ventral root of the zygomatic process of the maxilla.
Samples from BO and PE have a similar cranial architecture relative to specimens from northwestern Argentina, differing slightly from them in the quantitative characters.
Discussion
The present study provides mostly coincident results from quantitative and qualitative morphological traits, which allow differentiating the southern Argentinean samples of Lagidium viscacia from those of northwestern Argentina, as well as separating both from L. peruanum (Figure 2C). The magnitude of these differences stands at the species level (cf. Ledesma et al. 2009; Spotorno and Patton 2015); the question that remains, however, regards the proper allocation of names to the southern and northwestern Argentinean forms.
Specimens from west-central (ME) and southern Argentina are characterized by grayish colorations (CH, RN, SO; except NQ which is yellowish gray), with well- marked and usually broad dorsal stripes, nasals expanded anteriorly and narrow dorsal roots of the zygomatic process of the maxilla. Four nominal taxa traditionally linked with viscacia were described from southern Argentina: moreni Thomas 1897, boxiThomas 1921, sarae Thomas and St. Leger 1926, and somuncurensis Crespo 1963 (Figure 2B). Hayman (in Ellerman 1940), Pearson (1995) and Spotorno et al. (2004) used boxi as the appropriate name for southern Argentinean and Chilean samples. Within this context, the status of moreni, included by Hayman (in Ellerman 1940) under his concept of viscacia (against Osgood 1943:141, which suggested that this taxon would be closely allied to boxi) should be adequately addressed, since this name precedes boxi. The type locality of moreni is unclear, a fact that restrains taxonomic assessments. Thomas (1897: 466) first referred it to “hills near Chubut, Eastern Patagonia,” but subsequently considered it as “unknown, as ‘Chubut’ is a province of considerable size, and there is no evidence as to the detailed location where the specimen was obtained” (Thomas 1921: 181). Lagidium is broadly distributed on western and central Chubut province, with some isolated population reaching its easternmost locality record near -65° W (Chebez et al. 2014). The specimens studied from Chubut and other areas of southern Argentina formed close clusters in the multivariate space, suggesting that only a single species is present in this area.
Most of the samples from northwestern Argentina (e. g., JU, LR, SA, SJ, TU) are characterized by yellowish-gray colorations, with diffuse to well-marked dorsal stripes, nasals not expanded anteriorly, and a broad dorsal root of the zygomatic process of the maxilla. At least five nominal forms were described from northwestern Argentina (i. e., tucumana Thomas 1907, vulcani Thomas 1919, lockwoodi Thomas 1919, famatinae Thomas 1920, tontalis Thomas 1921; Figure 2B). Osgood (1943) argued that “The physical conditions under which the animals [in reference to these nominal forms] live in this region are fairly uniform and one finds it difficult to accept the assumption that all these names are well founded…it seems desirable to reduce all of these names to subspecific status.” The cluster of specimens from western Bolivia in the multivariate space is close to those of northwestern Argentina and share a similar cranial architecture. However, both clusters differ in coloration, which was mostly grayish for those from western Bolivia. At least three nominal forms were described for Bolivia: cuscusThomas 1907, luteaThomas 1907, and perluteaThomas 1907 (Figure 2B). Anderson (1997) recognized a single species in Bolivia (L. viscacia), with three subspecies (L. v. cuscus, L. v. lutea, L. v. perlutea), mapping several localities geographically close from the hypothesized distribution of L. peruanum in the Peruvian Highlands. In turn, Osgood (1943) referred populations from northern Chile and adjoining Bolivian areas as L. v. cuvieri, suggesting their potential synonymy with lutea. With the evidences currently available, it is unclear whether those populations in northwestern Argentina and western Bolivia correspond to a single or several species, or whether they belong to L. viscacia s. s. (as some molecular evidences suggest; see the discussion below).
From the above, it is clear that the taxonomic scenario within Lagidium remains poorly defined, especially for populations towards the north-central portion of their distribution. This situation is shared by other genera of caviomorph rodents, in which discrete morphological differentiation in cranial features is apparently limited (e. g., Dasyprocta [e. g., Teta and Lucero 2016], Galea [e. g., Bezerra 2008]). Overall, our results contradicts the traditional view that considers L. viscacia as a largely distributed rodent species (e. g.,Spotorno and Patton 2015). The evidence reported here, plus the one derived from of previous molecular approaches (e. g., Spotorno et al. 2004; Ledesma et al. 2009) strongly suggest that this taxon, as currently delimited, encompasses two or perhaps more species.
Our analysis, although preliminary, demonstrates that the Argentinean populations of L. viscacia correspond at least to two different species based on morphological and molecular evidences (cf. Ledesma et al. 2009). Those populations from westcentral and southern Argentina (and possibly those on adjoining areas of southern Chile) could be preliminarily recognized as L. moreni, as discussed above. According to genetic data, this species is sister to L. wolffsohni, which is found farther to the south in montane areas of Argentina (Santa Cruz province) and Chile. Although we have not analyzed any specimens of L. wolffsohni, we preliminarily considered this species as distinct based on its striking orange coloration, short ears and large overall size (cf. Hayman [in Ellerman 1940]). As regards the samples from northwestern Argentina, the available evidence is inconclusive, since some molecular-based studies linked some populations in Jujuy (Argentina) and Antofagasta (Chile) to L. viscacia s. s. (specimens from central Chile; Spotorno et al. 2004), while data allocated these populations as a sister to a clade formed by the populations recognized here as L. moreni plus L. wolffsohni (Ledesma et al. 2009). Thus, without analyzing the topotypes of all nominal forms as well as adequate samples across the entire distributional range of the genus, it is premature to put forward any formal taxonomic proposal; we prefer to maintain those populations from northwestern Argentina, western Boliva and central Chile under L. viscacia.
Nominal forms such as pallipes Bennett 1835 and viatorumThomas 1921 were not included in our study, nor were specimens from the northern Mendoza province, an area where the southern and northwestern groups could be in contact. Unfortunately, the only specimen examined from southern Mendoza province lacked its skull. Additional data, including molecular evidence, is much needed in order to clearly demarcate the distributional boundaries between species, especially in highly complex topographical areas such as the high Andean of northwestern Argentina, western Bolivia and northern Chile











 nueva página del texto (beta)
nueva página del texto (beta)


