Introduction
Terrestrial mammalian herbivores play key roles in terrestrial ecosystems (Ripple et al. 2015). Ungulates in particular shape the structure and function of landscapes they inhabit, via trampling, consumption of plants, seeds dispersal and nutrient cycling (Wright et al. 2007a; Ripple et al. 2015), and are therefore often referred to as 'ecosystem engineers'. Moreover, they constitute an important source of food for predators and for humans (Naranjo et al. 2010; Altrichter et al. 2012; Ripple et al. 2015). Currently, a large number of ungulates species worldwide are threatened, mostly due to unsustainable hunting for bushmeat and habitat degradation (i. e. deforestation and human encroachment; Wright et al. 2007b; Van Vliet et al. 2014; Ripple et al. 2015). This results in a global decline of ungulates throughout their range which may have dramatic consequences on the integrity of the ecosystems they occur in (Wright et al. 2007a, b; Galetti et al. 2015a; Ripple et al. 2015).
Panama is no exception to this pattern: the country has experienced a high economic development in the last decades that may have consequences on the environment (Heckadon-Moreno 1993). For example, it has lost 14.3 % of its forest cover between 1990 and 2010 (Food and Agriculture Organization of the United Nations 2010), a loss that is often associated with other threats such as poaching and human encroachment that affect its fauna (Wright et al. 2000; Urquiza-Haas et al. 2009), including five ungulates species - the Baird's tapir (Tapirus bairdii), the white-lipped peccary (Tayassu pecari), the collared peccary (Pecari tajacu), the white-tailed deer (Odocoileus virginianus) and the red brocket deer (Mazama temama). Two of these species - the white-lipped peccary and the tapir - are particularly vulnerable and less adaptable than the other species in disturbed habitat, and therefore constitute indicators of the integrity of the ecosystem (Reyna-Hurtado and Tanner 2005; Naranjo and Bodmer 2007; Altrichter et al. 2012; Garcia-Marmolejo et al. 2015). The isthmus of Panama, physically narrow, is an important portion of the Mesoamerican Biological Corridor (MBC; DeClerck et al. 2010), a project that seeks to connect natural protected areas from Southern Mexico to Panama. As the ungulates species that Panama harbors span from North- or Central America to South America, the isthmus is essential in allowing movement and gene flow across the MBC to eventually maintain viable metapopulations of these species.
Protected areas are widely assumed to be effective in preserving the integrity of species composition in a region (Bruner et al. 2001; Le Saout et al. 2013; Beaudrot et al. 2006), because they generally comprise large blocks of habitat which some species require to maintain healthy populations (e. g. the white-lipped peccaries), and are usually less exposed to hunting pressure. In Panama, 22 % of the land area is protected (Food and Agriculture Organization of the United Nations 2010). Whether this protection is effective, however, is unknown. Small-scale wildlife population assessments carried out by the Ministry of the Environment (previously ANAM) in several of these protected sites had low sampling effort and mostly based on line-transects or interviews (see reports on www.miambiente.gob.pa). Interview-based information from local people reports the presence of all five species of ungulates in the majority of the forests of Panama decades ago (see reports on www.miambiente.gob.pa). More recently, few studies suggested the current degradation of the terrestrial mammal communities in both protected and unprotected areas of Central Panama (Meyer et al. 2015), and the uneven distribution of two endangered ungulate species, the tapir and the white-lipped peccary, along the forests of Panama (Meyer et al. 2013; Moreno and Meyer 2014). Nevertheless, studies evaluating the effectiveness of these protected areas to maintain intact ungulate assemblage along Panama and part of the MBC in a systematic manner remain scarce, which may result in poor or ineffective wildlife management and conservation measures.
In this study, we compiled camera trapping data and determined the occurrence and abundance of the ungulate species in several protected forests scattered along Panama to assess if they still supported intact ungulates assemblage. The sites surveyed were all covered of moist tropical forests with similar structure and temperature, and did not vary much in their topography and rainfall. Although most sites were relatively large, and/or contiguous to forested areas, their size varied quite substantially. We expected a positive correlation between ungulate species abundance and the size of the park, as some ungulate species, especially the white-lipped peccary, require large areas to survive (Reyna-Hurtado et al. 2009; Altrichter et al. 2012). Moreover, we expected the ungulate assemblages to be more impoverished in Central Panama than in the other sites because the mammal community of the region is known to be disturbed partly due to the high human density (Meyer et al. 2015).
Material and methods
Study area. Panama lies in the moist tropics with an average annual temperature ranging from 22 - 31 °C. The dry season runs from late December to early May, and the annual rainfall amounts 1,700 - 4,000 mm on the Pacific and Atlantic coasts (Ibáñez et al. 2002). Most forests in Panama are well drained, with closed canopy and a dense understory of tree saplings, palms and lianas (Condit et al. 2001), and are defined by Holdridge (1967) as evergreen moist or wet forests which are structurally not much different from each other (Condit et al. 2001). Large-scale disturbances such as hurricanes or fire do not occur. There are 53 terrestrial protected areas (henceforth referred to as "parks") in Panama covering 22 % of the land territory. Of the 43 % of the land that remains forested, 44 % is under protection (Food and Agriculture Organization of the United Nations 2010). Outside of the forests in the parks, the country is a mosaic of both old-growth and secondary forest patches surrounded by agriculture, cattle grasslands and human settlements (Condit et al. 2001).
We compiled information on ungulates obtained by camera trapping surveys in 13 parks scattered from the Western to the Eastern extremes of the Isthmus; all but one (Cerro Hoya, National Park [NP]) are part of the Mesoamerican Biological Corridor (Figure 1, Table 1). We also presented information of Cerro Hoya (Fort and Nielsen 2012) because it is the last large remnant of forest in the Peninsula de Azuero. Barro Colorado Island (BCI) is part of Barro Colorado Natural Monument (BCNM), and was declared protected in 1923. The majority of the other parks were created between 1976 and 1997. Three parks were created more recently, in 2001 (PN Santa Fé), 2009 (Donoso Multiple Use Protected Area) and 2010 (Damani Guariviara Ramsar Site). Data sources were publications, the database of the Tropical Ecology Assessment and Monitoring (TEAM) Network (data set identifier ID: 20160114072908_2173), and our own field studies (Table 1).

Figure 1 Isthmus of Panama (A) and inset of Central Panama (B) with protected areas (black boundaries) that were surveyed and the locations of the camera trapping polygons (grey circles with dots). Grey shading represents primary and mature secondary forests (ANAM 2000).
Table 1 Study sites characteristics and design of the camera-trapping surveys. Sites are listed following an East-West longitudinal gradient.
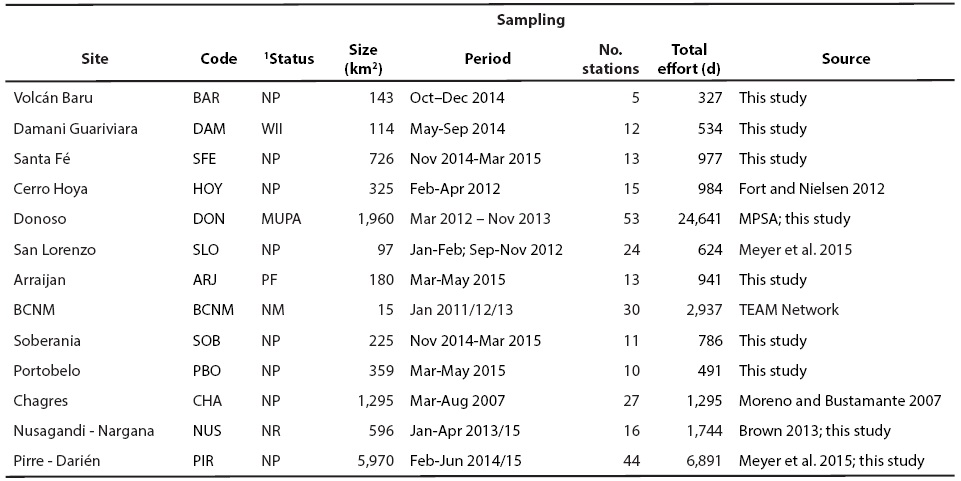
1NP = National Park, WII = Ramsar Site Wetland of International Importance, MUPA = Multiple Use Protected Area, PF = Protected Forest, NM = Natural Monument, NR = Nature Reserve.
Camera trap surveys. Camera traps have proven robust and reliable for surveying rare and cryptic species of tropical terrestrial mammals (Tobler et al. 2008). All surveys used unbaited camera traps deployed in a subset of the area because the sites were large (Table 1). With the exception of BCNM, all camera-trapping surveys were designed to maximize jaguars captures, i. e. camera traps (Cuddeback Inc., WI) were set up in pairs along trails and ridges, interspaced 0.8 to 1.5 km for assuring independent events. The survey in BCNM followed the protocol of the TEAM Network (Jansen et al. 2014) with the objective to randomly capture any terrestrial mammal species. Single camera traps (PC 900, Reconyx Inc, WI) were placed off-trail at 30 pre-defined locations in a grid with 1.4 km interspacing. We assumed that the probability of capturing ungulates was the same in spite of the different sampling design.
Photos of BCNM were processed in DeskTEAM (Fegraus et al. 2011) and all other photos were processed and annotated in Excel 2007 (Microsoft Corporation, Redmond WA, USA). Photos were manually grouped into sequences that represented the same visit of an animal or group of animals (i. e. peccary species) in a two hour-interval, which triggered the same camera one or multiple times depending on movement speed and residence time. This filter assured that visit events were independent despite the fact that some species spent a long time in front of the camera (i. e. peccaries). The total number of individuals was estimated when a group of peccaries triggered the cameras. For subsequent analyses, we calculated the capture rates for species, a measure of relative abundance, as the number of visits photographed per 100 trap days. This standardization allowed comparing the capture rate between sites in spite of differences in sampling effort. We assumed that for all ungulate species, the number of visits that the cameras recorded is proportional to the local density of the species, i. e. cameras will record an ungulate species more often where it is more abundant (Rovero and Marshall 2009).
Sampling effort varied from 327 to 24,641 trapping days (Volcan Barú and Donoso respectively; Table 1). We produced rarefaction curves that were fitted to sample days in estimateS ver. 9.10 (Colwell 2013), to calculate total species richness. The curves leveled off for all sites except Volcan Barú (Figure 2), so we assumed that the majority of the species were recorded in our surveys (Gotelli and Colwell 2011) and that it would take a significantly larger effort to record additional rare species.

Figure 2 Rarefaction curves based on camera trapping data in protected sites of Panama with moderate (A) and large (B) sampling effort. See Table 1 for sites codes. Cerro Hoya curve is not shown because it was not available from the source (Fort and Nielsen 2014) and we did not have the rwa data to produce the curves ourselves.
Data analysis. The capture rate of each ungulate species was compared between sites with a Principal Component Analysis (PCA) on the species per sites matrix, centered by species, using the software CANOCO 5 (Braak and Smilauer 2002) with square-root transformed detection rates. We used the Spearman rank correlation coefficient (rs) to test for a correlation between the abundance index of each ungulate species and the size of the park because residuals of species abundance were not normal.
Results
The collared peccary was the only species captured in all the sites surveyed (Table 2). The white-lipped peccary, in contrast, was recorded in just two of the sites, Pirre-Darién and Donoso (Table 2). The second most frequently captured species was the red brocket deer (nine sites), followed by the tapir and the white-tailed deer (seven and six sites respectively). The five species were recorded only in Donoso that had the highest sampling effort, whereas Damani was the site where the fewest ungulate species (one species) was captured. Camera traps recorded two species in four sites, three species in five sites and four species in the two sites of BCNM and Pirre-Darien (Table 2). Two to four species were captured in each site of Central Panama (Table 2).
Table 2 Capture rate of ungulate species recorded by camera traps across 13 protected forests in Panama and their local conservation status. - = not detected in our survey. See Table 1 for source of the data.

1 Conservation status in Panama based on ANAM (2008).
*Sites in Central Panama
The ungulates assemblages varied widely among the parks (Table 2; Figure 3). The first two axes of the ordination diagram explained 90.8 % of the total variation in the composition of the ungulate assemblage. The first axis (74.7 % of the total variation) explained the variation in ungulate assemblage between sites in central Panama and the other sites, and was related to the abundance of collared peccary and the two deer species. The abundance of these species was highest in the small and highly protected BCNM, and in Soberania and Arraijan. The second axis (16.1 % of the total variation) explained the differences between the large site of Darién and the smaller and much more disturbed sites of Cerro Hoya, San Lorenzo, Arraijan and Damani, and was related to the abundance of the white-lipped peccary and the tapir, two large frugivorous species that require large tracts of contiguous and undisturbed forest and that are locally endangered. While relatively abundant in Darién, they were rare or not detected in most other sites. Volcan Barú and Nusagandi were clustered together which indicates that they were similar in terms of ungulates species. Likewise, Chagres, Santa Fé, Portobelo and Donoso were also similar to each other.

Figure 3 Results of a Principal Component Analysis of ungulate assemblages, based on capture rates by camera traps at 13 protected sites in Panama. Arrows indicate the direction of maximum change in relative abundance across the ordination plot, where arrow length is proportional to the change and to the abundance. Sites that are close together are more similar in ungulate species composition and abundance. See table 1 for site codes.
Tapirs and white-lipped peccaries were more abundant in larger parks (rstapir = 0.67, P = 0.01 and rsWL peccary = 0.63, P = 0.02), while there was no significant correlation between the abundance of the other species and the size of the parks.
Discussion
Protected areas are widely assumed to effectively protect wildlife populations, especially when they are large. This study used camera-trapping surveys to evaluate the effectiveness of the protected areas to preserve ungulate assemblages in Panama. Our hypothesis that the ungulate assemblages were going to be impoverished in Central Panama was supported by the results. However, we unexpectedly found that the majority of the sites, including some large ones, harbored just two or three of the five ungulate species known for Panama, and our second hypothesis was not sustained.
The only species that was present in every site was the collared peccary, while the red brocket deer was captured in the majority of the sites as well. These two species have been documented to cope relatively well in disturbed landscapes with high level of hunting in other tropical regions while being adaptable to preserved areas as well (Peres 1996; Reyna-Hurtado and Tanner 2007; Tejeda-Cruz et al. 2009). The white-tailed deer was detected in just half of our sites, most in Central Panama, a region that is disturbed, fragmented and with a high hunting pressure (Wright et al. 2000; Meyer et al. 2015). Reyna-Hurtado and Tanner (2007) had found similar pattern in Calakmul, Mexico, where this species was more abundant in hunted sites. This is possibly explained by the habitat type as the species usually occurs in perturbed areas (Reyna-Hurtado and Tanner 2005; Garcia-Marmolejo et al. 2015). Interestingly, the collared peccary, the red-brocket deer and the white-tailed deer were much more abundant in sites of Central Panama where apex predators are very rare (Meyer et al. 2015), which suggests release from top-down control (Terborgh et al. 2001).
Tapirs and white-lipped peccaries in contrast, are two elusive species particularly sensitive to disturbance, and were infrequently recorded, even in relatively large protected areas. Tapirs were photo-captured in half of the sites surveyed that were also the largest with the exception of BCNM and Volcan Barú, even though they have relatively small home ranges (Foerster and Vaughan 2002). Volcan Barú is directly connected to the International Park of La Amistad that we did not survey but that is very large, remote, and known to harbor tapirs (Meyer et al. 2013). BCNM is the only site in central Panama that still harbors a resident population of tapirs, as a small number was reintroduced in the 1950´s after the species was extirpated (Terwilliger 1978) and nowadays the site benefits from a very high level of protection (Meyer et al. 2013). Although occasionally poached, tapir´s meat is not as much appreciated as peccary or deer species in Panama (Meyer et al. 2013) and is therefore not actively hunted; instead, they seem to particularly suffer from human encroachment which is common and uncontrolled in most parks in Panama, particularly through illegal logging and forest conversion to cattle pastures (pers. obs.). This pattern was supported by the higher capture rate in camera stations that were placed very remotely inside the parks where human access is difficult rather than in the buffer area (R. Moreno and N. Meyer, unpub. data). As the proportion of core area usually increase with the size of the area, it may explain why the abundance of tapirs was positively correlated to park size. The white-lipped peccary on the other hand was almost not recorded except for Pirre-Darién that still present extensive tracts of relatively undisturbed forest, and Donoso, a site that harbors the entire large-sized terrestrial mammal community of Panama (R. Moreno, unpub. data). However, in that latter site the capture rate was very low in spite of the substantial trapping effort, highlighting its rarity in the region. A finding that is similar in other sites where we did not photograph the species in our survey even though there is evidence of their occurrence, namely in Portobelo (H. Esser, pers. com.; Meyer et al. 2015), Nusagandi and Chagres (Moreno and Meyer 2014). A considerable larger sampling effort over a longer period of time would increase the probability of photographing the species in sites where they occur at very low density. White-lipped peccaries are habitat specialists, live in large groups and move in non-seasonally patterns, therefore require large continuous forests to maintain viable population (Peres 1996; Reyna-Hurtado et al. 2009). Nevertheless, most of the parks surveyed are relatively large and used to harbor white-lipped peccaries decades ago until they became over hunted (Moreno and Meyer 2014). Poaching remains the principal threat for white-lipped peccary because it constitutes a favorite game species, and reports of culling of a large proportion of herd-members, up to > 20 individuals at a single time are not unusual (Moreno and Meyer 2014). Such harvest practices contribute to drive the species to local extinction in many sites (Moreno and Meyer 2014) but also reveal the lack of protection in many parks.
The difference in ungulate detections between protected areas may not be related to park size per se, but rather to the surrounding fragmentation and anthropogenic activities (Ahumada et al. 2011). The geographic isolation of some parks to other source areas, as is the case of Cerro Hoya, may impede the recolonization by the species after extirpation. The situation looks particularly worrisome for tapirs and white-lipped peccaries as bushmeat trade studies in the Neotropics uncovered the magnitude of the phenomenon and revealed that hunting is often unsustainable for large-bodied species (Smith 2008; Naranjo et al. 2010; Van Vliet et al. 2014).
The non-detection of a specific species in a given site does not necessarily imply it is absent since rare species require a high sampling effort to be detected, as illustrated by the white-lipped peccary. The sampling effort varied substantially in our survey and may have been too low in some sites to detect species occurring at very low density, but our results highlight the rarity of such species. There was also a strong variation in the abundance of the species among the sites, potentially making some species functionally extinct in various sites. Functional extinction occurs when a species becomes too rare to fulfill its ecological role (Altrichter et al. 2012; Galetti et al. 2015b). As such, the pattern observed in our study constitutes an issue as the loss of large vertebrates due to hunting and habitat degradation, known as defaunation, may have important effects on the ecosystem functioning (Dirzo et al. 2014). Besides affecting large carnivores as a result of a decrease in preys (Ripple et al. 2015), the depletion of large frugivorous species such as tapir and white-lipped peccary in particular, have consequences on the dispersal and germination of seeds that can lead to significant changes in the vegetation structure and composition, further impacting species at a lower trophic level (Stoner et al. 2007; Wright et al. 2007a,b Galetti et al. 2015b). The effects of large vertebrates loss on seeds dispersal have been largely documented on BCI and in Soberania NP (e. g. Wright et al. 2000; Wright et al. 2007b), but would merit further investigation in the remaining sites composed of large tracts of primary forests where large seed dispersers species were rarely photographed.
Conclusion
We studied the effectiveness of protected areas to preserve the ungulate assemblages. Our results indicated a global impoverishment of the ungulates assemblage in several protected sites across Panama, and suggest that not all protected areas are effectively maintaining ungulate species. Increasing the surveys sampling efforts and monitoring a higher number of parks will contribute to reduce false absence, since some rare species, the tapir and white-lipped peccary especially, may not always have been captured in some of the sites in spite of their presence. Moreover, it is important to determine the factors driving the pattern observed and to what extent to help reversing this trend. For example, it is relevant to quantify the impact of bushmeat harvest together with the species population size in order to determine sustainable level of hunting at the local scale (Naranjo and Bodmer 2007).











 nueva página del texto (beta)
nueva página del texto (beta)


