The Ebola virus is found in several non-human and unrelated mammalian species; some organisms become sick with this illness but others do not. This fact complicates the determination of Ebola transmission routes. Bats are frequently a vector in the transmission of diseases to wild mammals and humans. However, the hypothesis about the transmission routes that relate wildlife species and humans are generally inconclusive. The transmission of the Ebola virus via body fluids of infected animals is one of the possibilities. This review proposes a "rain" of fruit-saliva as a primary Ebola transmission pathway. During their feeding process, fruit bats consume the juice of fruits and spit out the fibrous pulp, urinating and defecating during foraging bouts. During the night, this process produces a constant "rain" of bat droppings that contain both the Ebola virus and nutriments such as fruit glycoproteins. Close contact within a social group would likely spread the virus from infected to not-infected individuals rapidly. Considering this "fruit rain" hypothesis, Ebola would spread to humans through the consumption of food contaminated with this infected fluid rain, in addition to the direct ingestion of contaminated animals.
Currently there is not a clear understanding of how the Ebola virus is transmitted among wild reservoirs and from them to humans. The virus has been found in several non-human and unrelated mammalian species. This fact made even harder to determine Ebola transmission routes. Evidence currently available suggests that this disease is spread to humans either from one person to another or through an intermediate host (Jarman 1974). Several different mammalian species are known to host the virus, including fruit-eating bats of the Old-World family Pteropodidae; non-human primates such as chimpanzees and gorillas; duikers, which are small forest antelopes; and forest pigs (Sanchez et al. 1993; Newing 2000; Daszak 2010). Bats are apparently unaffected by the virus but serve as a vector in the transmission pathway (Leroy et al. 2004), while other mammals such as gorillas and chimpanzees succumb to the disease (Leroy et al. 2009). Members of the Ebola group of viruses have been found in Asia and Africa, but to date Africa is the area where wild mammals and humans have been infected (WHO 2014). Mortality rates in non-bat species are exceedingly high, being above 90% in humans (Wong et al. 2012). HIV/AIDS, SARS, and AH1N1 are other viruses that originated in wild mammals and became highly infectious diseases in humans (Kühl et al. 2011).
The hypotheses linking bats, other non-human mammals, and humans in the Ebola transmission pathway are inconclusive. However, all non-human species that have been recorded to host the virus -- either displaying clinical signs of the disease or not -- share two common traits: they are both frugivorous and highly social. Moreover, Ebola outbreaks in humans tend to be preceded by wildlife deaths due to infection (Sanchez et al. 1993). One route of Ebola transmission is throughout bodily fluids of infected animals. Bausch et al. (2007) conclude that during an acute Ebola outbreak, the virus is present in a wide variety of bodily fluids, but when those fluids are isolated, the risk of transmission is low. Therefore, we (Alvarez-Castañeda, pers. Communication) propose a fruit-saliva route as a major transmission pathway from wild mammals to humans (Figure 1). This hypothesis is supported by the observation that Ebola outbreaks in humans have occurred at the beginning of the tropical dry season (Sanchez et al. 1993), which corresponds to the end of the fruiting season of most tropical trees. A number of bat species tend to arrive to the same trees, and inter-species virus transmission (or even a simple antigenic stimulation) may potentially occur via infected saliva deposited on fruits (Pourrut et al. 2009). The transmission of diseases caused by virus such as Hendra and Nipah -- the former associated with respiratory and neurologic diseases in horses and humans, and the latter to an outbreak of encephalitis and respiratory disease in pigs and humans (Hooper et al. 2001) -- has been suggested to occur in this way. The proposed transmission route of those diseases is through the saliva of infected bats belonging to the Asian Pteropus species (Williamson et al. 1998; Pourrut et al. 2009) deposited on fruits subsequently consumed by humans (Pourrut et al. 2009). In the case of the ebolavirus, outbreaks in non-human primates have been associated with a weakened immune system after a period of food scarcity (Leroy et al. 2009). These primates may become infected by consuming fruits contaminated with blood and placentas from infected bats (Towner 2009). The ebolavirus enters host cells through a process mediated by glycoproteins (Jarman 1974) -- proteins associated with oligosaccharides that include fructose.
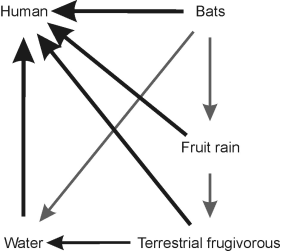
Figure 1 Via of transmission of the Ebola virus in the wild and to the humans. Terrestrial frugivorous are considered those species that feed on fruits very low or without climbing to the trees as chimpanzee, gorilla, duiker, and wild pigs)
Pteropodid bats are strictly frugivorous and most species roost and feed in large groups in the same or adjacent trees. Fruiting trees attract large numbers of bats and other frugivores, especially terrestrial species such as duikers and bush pigs. Considerable amounts of fruit fall to the ground as a result of natural ripening or dislodging during bat foraging among branches. Furthermore, bats do not consume large fruits whole, but crush the pulp, ingesting the juices and spitting out the fibrous pulp; they also urinate and defecate during foraging bouts. The Ebola virus has been found in fruit bat feces (Swanepoel et al. 1996). Thus, there is a near constant nighttime "rain" of whole fruits, partially eaten fruits, and ejected fruit pulp onto the forest floor, all potentially coated in bat saliva and urine/feces. Any Ebola-infected bat within a social group would add that virus to the fruit "rain," facilitating the transmission to other frugivores foraging on the ground. Also, the close physical contact between bats in a social group and the exchange of saliva during affiliative behaviors, which contributes to spread the virus from infected to non-infected individuals, hence increasing the incidence of the virus in the fruit "rain." Ebola is an RNA virus (Vogel 2014) with a limited infectious lifespan, and it cannot replicate in fruit. However, the high water content of fruits coupled with the high humidity typical of the tropical forest floor may extend the survival period of the virus and thus the time available to infect a new host. Carefully constructed experiments are required both to test the validity of the "fruit rain" hypothesis and to determine the lifespan of the virus under the conditions we propose.
Under our "fruit rain" hypothesis, the spread of Ebola to humans can occur in at least two different ways. The first is by direct human consumption of fruits of food contaminated with virus-containing bat saliva or urine/feces. A second mechanism would be through handling contaminated bushmeat during transportation or preparation for consumption, as emphasized in two recent articles in public sources (Maughan 2014, Flynnand and Scutti 2014). Duikers, bush pigs, and non-human primates are major elements of the bushmeat trade, and are thus a primary source of proteins for many African forest communities. Considering the above, a contributing factor to control the spread of Ebola and other diseases from animals to man relates to the improvement of adequate hygiene habits associated with food preparation, keeping in mind the critical role of bats as pollinators and predators of crop pests (Daszak 2010).











 nueva página del texto (beta)
nueva página del texto (beta)


