Introduction
Most investigated vertebrate species have at least one, and usually several, endemic herpesvirus. More than 200 herpesvirus species have been identified to date (Maness et al. 2011). When investigating the lineage of herpesvirus, many major subdivisions mirror the phylogenetic branching order of its hosts; thus, the herpesvirus appears to have co-diverged with its hosts and tends to be host-specific (Maness et al. 2011). Given the approximately 5,500 different species of mammals, it can be expected that the number of herpesviruses that exist in nature well exceeds the 200 species that have been identified thus far (Maness et al. 2011). All of the herpesviruses fall within the newly established taxonomic order Herpesvirales, which consists of three families: Herpesviridae (which includes the herpesviruses of mammals, reptiles, and birds), Alloherpesviridae (which includes the herpesviruses of fish and amphibians), and Malacoherpesviridae (bivalve herpesviruses (Changming et al. 2015)). Additionally, the family Herpesviridae contains three subfamilies: Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae (Davison 2010). Electron microscopy (EM) and serum neutralization, as well as analyses of other herpesvirus characteristics, were used to identify the alphaherpesvirus in lung and liver isolates (Goldstein et al. 2004). The presence of herpesviruses in cetaceans (the group of animals consisting of all of the porpoises, dolphins, and whales) has been recognized since the late 1980's using electron microscopy (EM) reports of herpesvirus-like particles in skin biopsies from beluga whales (Delphinapterus leucas, Martineau et al. 1988). Few cases of herpesvirus infection have been reported in cetaceans. Alphaherpesviruses have been associated with fatal systemic infections in Bottlenose dolphins (Tursiops truncatus), in a Cuvier's beaked whale (Ziphius cavirostris), and in cutaneous lesions in Bottlenose dolphins (Blanchard et al. 2001). Gammaherpesviruses have been identified in mucosal lesions in Bottlenose dolphins, Risso's dolphins (Grampus griseus), dwarf sperm whales (Kogiasima) and Blainville's beaked whales (Mesoplodon densirostris; Smolarek et al. 2006; Bossart 2007; Van Elk et al. 2009). In addition, non-purulent encephalitis associated with herpesvirus infection has been described in a harbour porpoise (Phocoena phocoena). In the first report of herpesvirus infection in Mediterranean Sea cetaceans, eight different Herpesvirus sequences were found in five of eight Striped dolphins (Stenella coeruleoalba) that were also infected with Morbillivirus(Soto et al. 2011). In these dolphins, no lesions were found that could be attributed to herpesvirus infection, and the pathogenic contribution of these herpesviruses remains unclear.
Herpesviruses (HV) have also been associated with encephalitis in a harbor porpoise (Phocoena phocoena) and with skin lesions of dusky dolphins (Lagenorhynchus obscurus) corroborated by EM (Soto et al. 2011). In addition to EM, immunoperoxidase staining (Soto et al. 2011), serum neutralization and enzyme-labeled immunosorbent assays have been used as indicators for the presence of herpesviruses in cetaceans (Van Elk et al. 2009). In this report, we document the presence of herpesvirus in free-ranging dolphins from Mexico in order to obtain information about the health status in this population in the southern Gulf of Mexico support future stranding events causes.
Material and Methods
Bottlenose dolphins were caught from and sampled in the Terminos lagoon, Campeche, Mexico, in the Southern Gulf of Mexico (18° 23' - 18° 52' latitude N and -91° 10' to -91° 52' longitude W) under the capturing license SGPA/DGVS 09924/10 SEMARNAT, following at all times the guidelines for the use and care of experimental animals, and NOM 062, with each procedure being supervised by a veterinarian. The blood samples were collected from the caudal vein using a Vacutainer(tm) system, exudates samples (vaginal, preputial or blowhole) were collected using sterile swabs, and the skin samples were collected using a needle Acu-Punch Kit RM (Acuderm(tm)).
Six blowholes and three vaginal and four preputial discharges samples were collected for this study. Samples of viral isolates were frozen in liquid nitrogen and thawed at 37 °C, repeating this procedure five times, and were then filtered through 0.2 µm Millipore(tm) membranes. A 0.5-ml aliquot was inoculated directly into 12-well microplates (NUNC(tm)) containing cells at 70 % confluence in MEM medium with 3 to 5 % newborn calf serum depending on the cell line. The plates were incubated in a CO2 atmosphere for five days and frozen and thawed 3 times, after which 1 ml was used to infect another cell culture flask; this step was repeated 3 times. The cells showed cytopathic and cytolytic effects.
The following immortalized cell lines were used: Madine-Darby Canine Kidney (MDCK), Bovine Kidney (MDBK) and African green monkey kidney (Vero). All of the immortalized cell lines were obtained from the American Type Culture Collection.
For the immunofluorescence assay, 12-well NUNC plates were prepared with a coverslip in the bottom, cells were grown to 70 % confluence, and the supernatants of the infected cell cultures were used to infect the cells on the coverslips, which were incubated for 48 - 72 hr. The coverslips were removed and fixed in pure acetone for 10 minutes at room temperature. The slides were stained with VMRD(tm) Canine Herpesvirus direct FA conjugate according to the manufacturer's recommendations. Other slides were simultaneously stained using the Diff-Quick Stain Kit (Fisher Scientific(tm)).
Herpesvirus DNA was amplified using published degenerate primers designed to target a region of the DNA polymerase gene of herpesviruses that corresponds to highly conserved amino acid motifs (Smolarek et al. 2006). These primers direct the amplification of DNA polymerase gene fragments that are 215 to 235 bp in length for most herpesviruses and 315 bp in length for cytomegaloviruses. A nested PCR assay was performed using two forward and one reverse primer in the first reaction and one forward and one reverse primer in the second reaction. The primer sequences for the first reaction were DFA-5'- GAY TTY GCI AGY YTI TAY CC -3' (forward), ILK-5'- TCC TGG ACA AGC AGC ARI YSG CIM TIA A -3' (forward), KG1-5'- GTC TTG CTC ACC AGI TCI ACI CCY TT -3' (reverse). Primers for the second reaction were: TGV-5'- TGT AAC TCG GTG TAY GGI TTY ACI GGI GT -3'(forward), and IYG-5'- CAC AGA GTC CGT RTC ICC RTA IAT -3' (reverse). The total DNA that was extracted from the cell monolayers of the Madine-Darby canine kidney (MDCK) cultures that were infected with canine herpesvirus (CHV) was used as a positive template for the PCR. From the second PCR, 14 µl were resolved by horizontal gel electrophoresis in a 1 % agarose gel containing ethidium bromide (0.5 ug/ml), and the DNA fragments were visualized by UV light trans-illumination.
For electron microscopy, the virus culture samples were fixed with Karnofsky, and a drop was placed on two grids (Sigma Aldrich 62 µm, copper) for 20 minutes. Then, a drop of distilled water and a drop of 1 % phosphotungstic acid (AFT), pH 7.2, were added for one minute, and the excess dye was removed with filter paper.
The samples were dried at room temperature and were observed and photographed on a transmission electron microscope (JEOL(tm) JEM 100S). We used a microparticle size standard based on a monodisperse polystyrene (Sigma Aldrich, Fluka) standard particle size of 2 µm as a reference for describing the structures that were observed in the samples. Several measurements at the same amplification were performed, and the average was calculated.
Results
Of all six blowhole, three vaginal and four preputial secretion discharges samples in this study, we were able to isolate viruses compatible with herpesvirus in three samples (two respiratory and one vaginal discharge). These viruses showed a strong cytolytic effect on the MDBK cells and showed no effect on the other cell lines. The effect involved syncytia formation, the presence of inclusion bodies in the cells and cell lysis (Figure 1). All of the infected cell cultures showed strong fluorescence when stained with Canine Herpesvirus direct FA Conjugate (Figure 2).
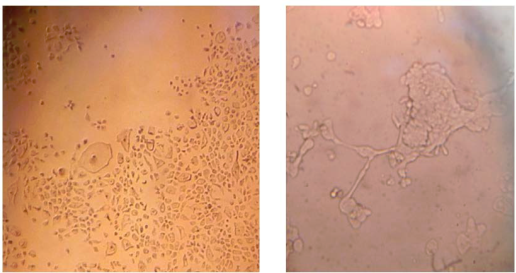
Figure 1 Infected MDBK cells at 48 hr (A) and at 72 hr (B). Cytopathic effects on the cells are seen (A), including syncytia formation (B). Picture A is 100x and picture B is 400x using an inverted microscope. No cytopathic effects were observed on the cells of the negative control. Other cytopathic effects were characterized by morphological changes, including cell rounding, swelling, and detachment.
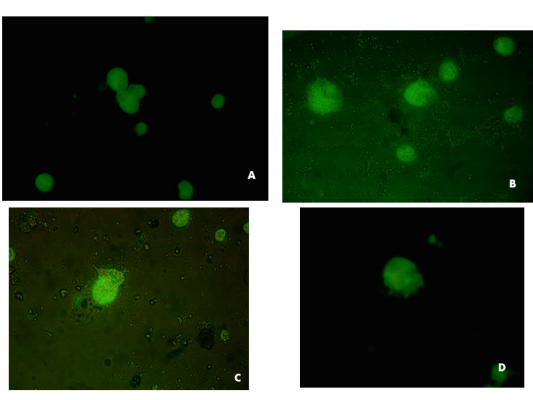
Figure 2 Canine Herpesvirus Direct FA, conjugated over infected MDBK cells at 48 hr. 400x. Respiratory sample 1(a) and sample 8 (b) and (c, d) sample 15 vaginal discharges.
Transmission electron microscopy revealed the presence of herpesvirus-like encapsidated particles. The average of the measurements was 92 nm for the virion and 196 nm for the complete virus with envelope (Figure 3), as previously reported for other dolphins herpesviruses (Rehtanz et al. 2012).
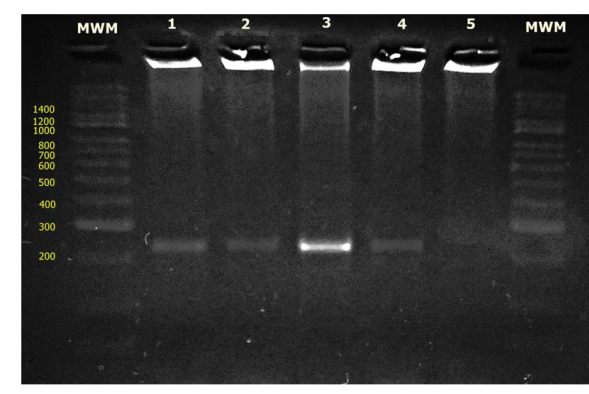
Figure 3 Gel electrophoresis of herpesvirus nested PCR products; MWM: Molecular Weight Marker, lane 1- positive control CHV-1, lane 2 - 001 (respiratory secretion), lane 3-008 (respiratory secretion), lane 4- 015 (vaginal discharge), lane 5- negative control.
When the PCR was performed using the whole DNA of the samples, amplification was not achieved. Nevertheless, the PCR that was performed using the DNA of isolates resulted in an amplification of 240 bp (Figure 3). The MDBK cells showed high permissibility for the development of the virus from the dolphins, possibly due to the phylogenetic association between dolphins and cattle; the observed effect corresponds to that described for alphaherpesviruses. Cytopathic characteristics,such as viral intranuclear inclusion bodies, syncytia formation and rapid cytolytic effects (3 - 5 days), were described for this group.
The immunologic cross-reactivity between the presumed isolated dolphin herpesvirus and the Canine Herpesvirus Direct FA conjugate suggests that the isolated virus is most likely an alphaherpesvirus.
Discussion
The capacity of herpesviruses to cause disease in cetaceans is unclear and may show variations depending on the conditions of different individuals and species. In a series of 128 samples of skin and mucosal lesions from 12 different cetacean species, as many as 11 different herpesvirus sequences were amplified (nine from 32 mucosal lesions and two from a total of 88 skin lesions), showing an apparently high association of herpesvirus infection with mucosal and skin lesions (Smolarek et al. 2006). However, systemic disease or internal lesions attributable to herpesvirus have been described only sporadically in cetaceans (Blanchard et al. 2001), one Cuvier's beaked whale and harbour porpoise. One disseminated herpesvirus infection was observed in two bottlenose dolphins with necrotizing lesions and eosinophilic intranuclear inclusion bodies in multiple organs (Van Elk et al. 2009). Previous studies have associated the presence of herpesvirus in the skin lesions of fatal systemic diseases in Atlantic bottlenose dolphins (Smolarek et al. 2006).
In cetaceans, the exhaled blowhole sputum can often be diagnostically valuable, as the blowhole possesses wide-bore airways through which the exchange of approximately 80 % of the lung volume occurs in one breath (Varela et al. 2007). In this work, five samples (2/6 blowhole, 2/3 vaginal and 1/4 preputial discharges) were observed, with a greater number of lymphocytes and plasmacells than normal (more than five per high-power field) but no evidence of inclusion bodies (results not shown). Chronic inflammation is typically represented by the presence of lymphocytes (small and large lymphocytes as well as plasma cells) and macrophages (Varela et al. 2007). The lymphocytes possibly belong to T cells, as has been described for other species (Patela et al. 2012). Despite several passes made in the different cell lines, the isolated virus was only able to produce cytopathic effects on the MDBK cells, different from the behavior of phocid gammaherpesviruses, which have been observed in different mammalian cell lines (Byron et al. 2007).
We could not subject to electron microscopy the exudate samples. However, herpesvirus particles were detected from the viral culture; while the amount of viral particles was high, whereas in electron microscopy there must be a minimum of 106 - 108 virus particles/ml in a sample to allow for detection (Curry et al. 2006).
We used the microscopic characteristics of herpesviruses for identification (Davison 2010). The electron microscope images showed a capsid structure that consists of a DNA core surrounded by an icosahedral (20-faceted) capsid consisting of 12 and 150 capsomeres. The capsid is embedded in a proteinaceous matrix called the tegument, which in turn is invested in a glycoprotein-containing lipid envelope.
Herpesviruses are more difficult to detect than other viruses in the tissues of marine animals, especially when the viral load is low and the viral type is not yet known. The viral load can be so low that proving the virus's presence may be impossible (Rehtanz et al. 2012). Thus, negative PCR results indicate a lower viral load in the samples associated with the presence of a greater proportion of lymphocytes in the cytology. It has been suggested that the presence of herpesviruses in the cervical secretions could even constitute a prognostic factor for cervical pathology in these dolphins or the establishment of a chronic viral infection. In this sense, two events are fundamental to the establishment of a chronic viral infection: first, the virus must evade sterilizing immunity (the complete elimination of a virus) and second, the immune system must adjust to the continuous presence of viral antigen-driven inflammatory responses in order to limit the viral replication to an acceptable level without untoward damage to permanently infected tissues. If the immune system cannot eliminate the virus, unrestrained immune attack on the virus antigen-bearing cells causes immunopathology. Thus, the down regulation of inflammation during chronic viral infections can result in decreased tissue damage, at least for noncytopathic viruses (Herbert et al. 2009). Moreover, it is rare that herpesvirus PCR sequences cannot be identified from a vertebrate species. The biology of these viruses is complex, and there has been some suggestion that they form, in essence, a 'viral normal flora' in their hosts (Tarlinton and Dunham 2011).(Figure 4)
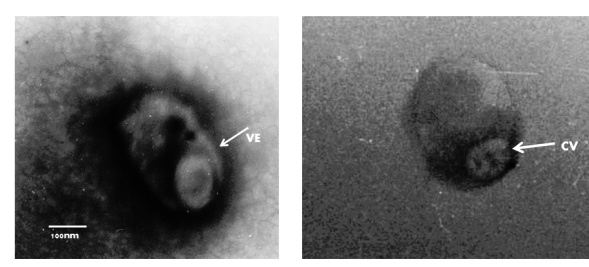
Figure 4 Negative staining electron microscopic examination of the MDBK cell culture. The sample was stained with 2 % phosphotungstic acid. The bars represent 100 nm. At a primary magnification of 30,000x. The capsid of virion (CV) appears to have a hexagonal outline (icosahedral morphology) and is surrounded by a limiting glycoprotein-containing lipid envelope (VE). The average of the measurements was 92 nm for the virion and 196 nm for the complete virus with envelope.
The positive results of viral isolation in the MDBK cells, the cytopathic and cytolytic effects in the MDBK cells observed at 48 hours, and the positive immunofluorescence, electron microscopic and PCR results of the viral isolates suggest the presence of an alphaherpesvirus (Davison 2010). This is the first report of the identification of the presence of a herpesvirus in free-ranging bottlenose dolphins in Mexico. Although this virus is not associated with any clinical disease, more intense studies must be performed, mainly on stranded cetaceans, to correlate the presence of the virus and the pathological findings. This conclusion is based on the examination of a relatively small number of cases, and further studies are recommended to determine the pathogenic potential of this herpesvirus











 nova página do texto(beta)
nova página do texto(beta)


