Introduction
Societies in mammal species are dynamic and complex (e. g. carnivores, primates, cetaceans), in which many of their members may interact and associate with other known or unknown individuals, forming distinctive assemblages (Goodall 1986). The structure of such social units is often affected by age, sex, reproductive status, hierarchy and kin selection (Beddia 2007); all these aspects may modify the behavior of individuals (Bräger 1993) and determine how the animals spend their time in certain areas, thus producing different patterns of residency, seasonality or migration that change their associations and the social structure of the population (Scott and Chivers 1990).
Dolphin societies or communities (sensuWells et al. 1987) are generally composed by individuals that inhabit the same general area and have frequent interactions with each other (Goodall 1986); thus marine mammalogists often use this term to describe assemblages of individuals of the same species, instead of assemblages of different species as referred in texts of classic ecological theory (Roughgarden 1989). Such aggregations are loose and fluent, where individual and genetic exchange may occur over time within the limits of the community (Wells et al. 1987).
Individual interactions are influenced by intrinsic factors such as the presence of recurrent associations (Connor et al. 2001), which in turn may be determined by extrinsic factors such as habitat variability (e. g. food abundance and availability, as well as natural and human-related threats (Lusseau et al. 2006; Quintana-Rizo 2006; Morteo and Hernández 2007; Morteo et al. 2012). Social and ecological pressures may dictate the temporality of the associations at different scales (McDonald and Carr 1989; Quintana-Rizzo 2006; Morteo et al. 2014). For instance, some species are known to maintain strong permanent and even multilevel associations such as killer whales (Orcinus orca) and sperm whales (Physeter microcephalus; Bräger et al. 1994; Whitehead et al. 2012), whereas other like the spinner dolphin (Stenella longirostris) are very loose (Chilvers and Corkeron 2002).
The social structure of coastal bottlenose dolphins (Tursiops truncatus) has been characterized as fission-fusion, involving small groups that constantly exchange individuals forming a wide range of social bonds (Goodall 1986; Wells et al. 1987; Connor et al. 1992). Several communities of the coastal form of this species are known to present segregation related to sex, age and reproductive status, thus the nature of their associations is highly variable (Wells et al. 1987; Wells 1991; Connor et al. 1992; Smolker et al. 1992; Connor et al. 2000; Quintana-Rizzo and Wells 2001; Morteo et al. 2014).
Living in social groups facilitates feeding, protecting against harassment, predation, enhances reproductive output, and promotes communication and learning (Bräger et al. 1994). Individuals may join or leave a group in response to the gains or losses of participating with the partners involved within a given social unit (Wrangham et al. 1993). Thus recording the activities of animal groups is useful to establish behavioral patterns in a specific habitat (Bräger 1993; Steiner 2011), but also to determine the current state of individuals within a community (Beddia 2007).
Coastal bottlenose dolphins inhabiting the waters off Alvarado in the state of Veracruz have been studied intermittently since 1993 (García 1995) and reliable data on individual identities has been collected since 2002 (Del Castillo 2010; Morteo et al. 2014). Many of these dolphins are known to associate and develop a range of activities, but little is known on how these animals interact and form groups, and if they do it with different purposes (Morteo 2011; García-Vital 2012). The goal of this study is to establish if school size is determined by the activities of the dolphins, and if individuals associate with specific partners to develop particular sets of activities.
Materials and methods
Study area. The Alvarado region is a shallow (< 20 m depth) open coastal habitat in the southwestern Gulf of Mexico, strongly influenced by river discharges (Figure 1); habitat modification is the major threat to the area (Del Castillo 2010; Morteo 2011; García-Vital 2012). It is the third largest coastal lagoon system in Mexico, and according to the National Institute of Fisheries (INAPESCA), it's the most important shrimp fishing ground in the state of Veracruz, which takes place year-around depending on weather and market demands. Sea surface temperature ranges from 20 to 32.5 °C, with an annual average of 27 °C. The regional climate is tropical with three seasons: a Dry season (March-June) with a significant reduction in average precipitation; a Rainy season (July-October), in which runoff from rivers into the adjacent lagoon and mangrove forest causes high organic matter and nutrient input into coastal waters; and a Windy season (November-February, locally known as "Nortes"), featuring strong winds (up to 80 km h-1) associated with the incursion of northern cold fronts, which may last several days. Fisheries are relevant to coastal dolphin populations due to the frequent adverse interactions between dolphins and local fisheries, causing incidental mortality of dolphins in nets (Morteo 2011; Morteo et al. 2012). Around 2000 fishermen were active in the area, most of which (75 - 85 %) operate in the lagoon and the rest operate in open waters. No official data is available on marine traffic or fishing effort and port facilities are dedicated to fishing, therefore there is no alternative commercial seagoing activity.
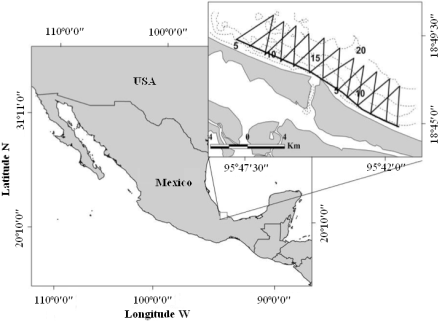
Figure 1 Study area and survey trajectories (bold lines). Dashed lines show depth contours every 5 m.
Surveys. Photographic line-transect surveys were conducted at least twice per month from October 2002 to September 2003 and from May 2006 to April 2009. The extent of the surveyed area was dependent on the duration of daily operations, and intended to maximize the chance of encountering coastal dolphins, based on their habitat preferences (Fazioli et al. 2006). Surveys were carried out at constant speed (15 - 18 km h-1) always in low swell conditions (sea state Beaufort ≤ 3, where wind speed < 15 km h-1) on board of a 7 m outboard fiberglass skiff (40 / 60 hp). When dolphins were sighted, the survey was paused to allow habituation (10 min) while their location was recorded using a GPS (Garmin eTrex Legend). Dolphins were then observed until their behavior was determined ad libitum (Altmann 1974) and classified into one of the most commonly used categories which were: 1) Feeding, 2) Socializing, 3) Traveling, 4) Avoiding, 5) Resting, and 6) Undetermined, following the literature (see Shane 1990; Bräger 1993; Chilvers and Corkeron 2002; Constantine et al. 2004; Steiner 2011). Subsequently, the group was approached with caution to avoid disturbing them, at the time that group size was estimated; we used an inclusive definition of group, consisting in all dolphins observed in apparent association, moving in the same direction and often, but not always, engaged in the same activity (Bräger et al. 1994); however, some groups included only one dolphin (Fazioli et al. 2006). Dolphins were followed until all dorsal fins were photographed or until they were lost from sight; we used SLR cameras, both analogical (Canon EOS Rebel 2000 with film Kodak Tri-X-pan ISO 400) and digital (Canon Rebel XT and Nikon D50) with 70 - 300 mm lenses. The survey was then resumed and the search proceeded until the study area was completed.
Group size and behavior. All sighting records from 2002 - 2003 and 2006 - 2009 (2002 - 2009 henceforth) were arranged into histograms to determine the most common types of aggregations and behaviors within the area. Average group size was computed for comparison, whereas differences among months, seasons and years were examined for both group size and behavior frequencies using non-parametric tests (α = 0.05). Also, a correspondence analysis was performed to explore the relation between group size and the behavioral categories using Statistica 7.0.
Photographic identification. Individual dolphins were identified by the marking patterns on their dorsal fins (Würsig and Jefferson 1990; Morteo 2011). Markings such as tooth rakes, scars, pigmentation marks, superficial wounds, and epiphytic organisms are temporary, thus these were considered unidentifiable and were excluded. Only dolphins with permanent and conspicuous markings were included, only if they were sighted in five or more survey days (Bräger et al. 1994; Félix 1997; Bejder et al. 1998; Rogers et al. 2004); thus, analyses were performed only for dolphins with a certain degree of residency.
Association patterns and behavioral diversity. Photographic data were also used to compute half-weight (i. e. controlled for sighting frequencies) coefficients of association (COA) for each dyad (pair of individuals; e. g. Smolker et al. 1992; Bräger et al. 1994; Félix 1997; Quintana-Rizzo and Wells 2001; Rogers et al. 2004) using SOCPROG 2.4 (Whitehead 2009). COA values range from zero for dolphins that are never seen together, to one for a pair that is always seen together. COA values were categorized as infrequent (0.0 - 0.2), casual (0.2 - 0.4), fair, (0.4 - 0.6), moderate (0.6 - 0.8) or strong (0.8 - 1.0; Smolker et al. 1992; Quintana-Rizzo and Wells 2001). Also, to prevent the occurrence of artificial dyads (i. e. individuals photographed together by chance due to our inclusive definition of group), a preferred/avoided partner assessment was developed through a permutation test (Smolker et al. 1992; Bejder et al. 1998; Gero et al. 2005).
The recorded behaviors were used to calculate a measure of diversity for the activities developed by all paired individuals; therefore, the Shannon-Wiener index was used as a proxy for each dyad; the latter was standardized to meet the COA range values (0 - 1) and renamed as activity diversity index (ADI). Since dolphin sightings containing more than two individuals would use the same behavioral records for the computation of the ADI in their respective dyads, the result would overestimate the contribution of each pair to the index (Hurlbert 1984); therefore, we randomly removed photographic data from individual dolphins until the statistical distribution of the ADI and the COA values stabilized. We then computed new COA and ADI in order to reduce the bias from the pooling fallacy (Machlis et al. 1985). Finally, the resulting COA and ADI matrices were analyzed using a Mantel's one tailed test, which measures the correlation between the values within two symmetrical matrices containing the data from all the possible combinations between individuals, throughout a randomization process (α = 0.05, Spearman correlation, Monte Carlo method and 10,000 permutations).
Results
Survey effort and group size. The Alvarado area was sampled on 80 photographic surveys accounting for 237 groups and 2,021 sighted dolphins. Average school size was 8.5 (s. d. = 8.6) but single animals (16.7 %) and pairs (16.3 %) accounted for almost a third of the recorded sightings (Figure 2). Groups were significantly larger in specific months (June and April, P < 0.01, Table 1), seasons (dry, P < 0.01, Table 1) and years (2002-3, 2007-8, P < 0.01, Table 1) across the study period.

Figure 2 Group size proportion for bottlenose dolphins sighted in the study area during 2002 - 2009 (n = 237).
Table 1 Group size (numbers) and prevalent behaviors of bottlenose dolphins observed within different periods in the study area (n = 237, * = P < 0.01).
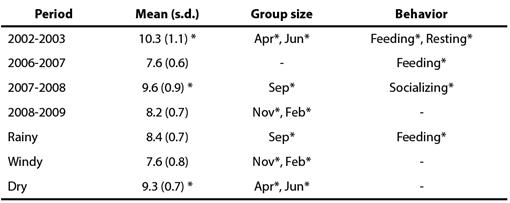
Behavior and group size. Behavioral records existed for 220 of the 237 sightings, where feeding was the most common activity in the area (29 %, χ2 = 15.22, P < 0.05), but this was true only for years 2002-2003 and 2006-2007 (P < 0.05, Table 1). Seasonal differences were observed only for the rainy months, were feeding was also significantly more frequent (χ2 = 23.05, P < 0.05).
Group size was arranged into class intervals according to its frequency in order to homogenize sample sizes (Figure 2). Correspondence between group size and behavior was significant within the studied period (χ2 = 69.47, P < 0.01) where the first two dimensions explained 76.1 % of the variance, and only three behavioral categories were associated to group size: dolphin pairs were mostly resting, whereas feeding aggregations were formed of four to six dolphins, and solitary individuals commonly showed evasion (Figure 3).
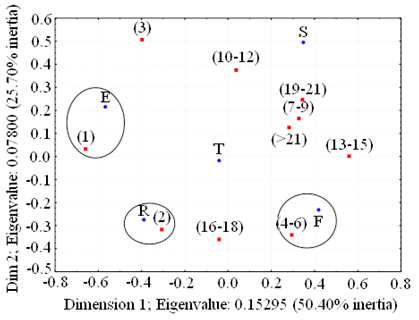
Figure 3 Correspondence analysis between activities and group class (size) for bottlenose dolphins sighted in the study area during 2002 - 2009. F = Feeding; T = Travel; E = Evasion S = Social; R = Rest (n = 220).
Association patterns and behavioral diversity. A total of 232 different dolphins were individually identified, but only 89 were sighted at least five times, and only one was recognized on up to 47 occasions. Association values (COA) changed annually (Figure 4), as the composition of the members within the community was very fluid, and only eight individuals were consistently identified over the years. The combination of these 89 individuals resulted in 3,915 possible dyads and 67.2 % of these were photographed at least once; however, the permutation test showed that only 237 pairs (6 %) were non-random (P < 0.05). From the latter, visual inspection of COA values showed that 11 % avoided each other (0.0 - 0.2), or met infrequently (0.2 - 0.4), whereas 68 % were moderately associated (0.4 - 0.6), and the remaining were either close (7 %; 0.6 - 0.8) or very close (0.8 - 1.0) partners (3 %; Figure 5).
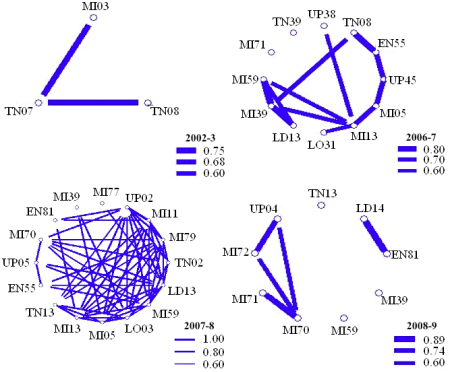
Figure 4 Annual sociograms for dolphins identified at least 5 times in the study area during 2002 - 2009. Line thickness shows the values for coefficients of association between dyads. Individuals with COA < 0.6 were removed (n = 27, n = 89), and also diagrams and line thickness were scaled differently to fit the figure.
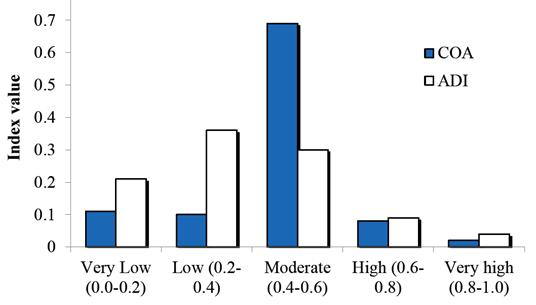
Figure 5 Proportion of non-random dyads by association (COA) and behavioral diversity (ADI) class in the study area during 2002 - 2009 (n = 237).
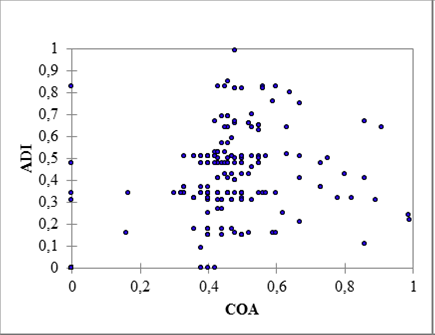
The diversity of activities (ADI) for each dyad showed a wide range of values, and its statistical distribution stabilized upon the random elimination of half of the records from the 237 significant pairs (Figure 5). Only 5 % of the dyads had very high diversity (0.8 - 1.0), 9 % had high diversity (0.6 - 0.8), 31 % were fairly diverse (0.4 - 0.6), 34 % developed a low diversity of activities (0.2 - 0.4), and 20.3 % of the dyads were selective (0.0 - 0.2, all null values corresponded to avoided partners, i. e. COA = 0; Figure 5).
The Mantel's test showed significant correlation between the COA and ADI matrices (r(AB) = 0.98, P < 0.01), thus the diversity of activities developed by each dyad increased significantly with their level of associations; however, a few instances deviated significantly from this pattern. For instance, 7.2 % of the dyads had moderate to very high associations but developed a low or very low diversity of activities (low-right corner in Figure 6), specifically feeding and socializing.
Discussion
Group size. Aggregations in animals are widely variable in size and composition, and their study is closely linked to the criteria used to define their limits (Shane et al. 1986; Wells et al. 1987; Shane 1990; Wells 1991; Smolker et al. 1992; Bejder et al. 1998; Whitehead 1999; Connor et al. 2000; Mareike 2003; Morteo 2011). The number of individuals in a group has been related to advantages while overcoming selective pressures, including but not limited to protection against harassment and predation, improving food acquisition and energy efficiency, thus leading to enhanced reproductive output (Wells et al. 1980; Shane et al. 1986; Mareike 2003).
Mean group size is often helpful to represent the optimal number of individuals for a community within a specific habitat (Würsig 1979), and the average group size found here (8.5 ± 8.6 s.d.) was similar to other coastal locations across the Gulf of Mexico, the Caribbean and the Atlantic (Shane et al. 1986; Wells et al. 1987; Scott and Chivers 1990; Delgado 2002; García 1995; Morteo and Hernández 2007; Hernández-Candelario 2009; Martínez-Serrano et al. 2011). However, since the statistical distribution was skewed towards smaller aggregations (i. e. one third of the sightings were composed of solitary individuals and pairs) despite our inclusive definition (Figure 2), this value seems inconclusive. Morteo et al. (2014) anticipated this for bottlenose dolphins within the same study area, and determined that many of these small aggregations were likely single sexed. This strongly suggests that the community may be divided into small social units that join in common activities (Campbell et al. 2002).
Behavior. The general behavioral pattern suggested for most bottlenose dolphin communities is: Travelling > Socialization or Feeding > Resting > Evasion (Morteo 2002). Feeding has been reported as a common activity in many dolphin communities (Shane 1990; Bräger 1993; Morteo 2002; Beddia 2007; Steiner 2011) and was the single most frequent activity (29%) within the studied period, especially in the rainy months (15.5 %). Maze and Würsig (1999) hypothesized that dolphins may use environmental cues such as continental water runoffs to congregate over shallow coastal areas close to rivers and lagoons, taking advantage of increased prey populations; in fact, coastal bottlenose dolphins in this and in neighboring locations are known to experience seasonal expansions in their distribution during this time of the year (Martínez-Serrano et al. 2011; Medellín-Ortiz 2012). Although the latter may seem to enhance the possibilities of interacting with other dolphins in larger aggregations, Morteo (2011) suggested that as food seems abundant and predators are scarce in this study area, large groups might not necessarily imply an advantage for foraging or protection. The statistical distribution for group size is consistent with this hypothesis and it also explains the correspondence between the feeding records and the groups composed by 4 - 6 dolphins (Figure 3), thus strengthening the notion of a small-unit based social structure.
Following the same hypothesis, solitary individuals were significantly associated to evasive behavior (17 % of recorded sightings, Figure 3), which may be due to the frequent and antagonistic interactions between dolphins and artisanal fisheries using gillnets in this location. Resident individuals face the inherent risk of being harassed, entangled or even killed, thus individuals rather than groups are more likely to evade detection by fishermen due to the extremely low visibility below the sea surface (Morteo et al. 2012). The latter also supports the argument that individuals in general gain larger benefits by aggregating in small groups (Figure 2) within this heavily fished area, and in extreme cases points to a "selfish strategy" as a mean for auto-preservation.
Habitat seasonality agreed with temporal differences found in both group size and behavior (Table 1; Del Castillo 2010; Martínez-Serrano et al. 2011; Morteo 2011; Medellín-Ortiz 2012); however, short-term habitat variability also seems to play an important role, since both of these variables were inconsistent throughout the studied period (Table 1). Morteo et al. (2012) pointed out the importance of boat traffic and fishing activities in the area, which seem to influence the presence and distribution of dolphins on a daily basis or even instantaneously (Constantine et al. 2004; Lusseau et al. 2006; Hernandez-Candelario 2009). Although many cetaceans seem to have habituated to certain levels of marine traffic, other evidence suggest that it may cause severe alterations in their behavior and in this case may promote the separation of group members, probably altering the social bonds within the community (Constantine et al. 2004; Morteo et al. 2012; Morteo et al. 2014).
Association patterns and behavioral diversity. A social organization is defined by the relations and interactions among individuals within the sampled population (Chapman et al. 1995). Societies are believed to gain from all the variety in their associations (Dunbar 1989); however, even in fission-fusion societies, partners could be selected to maximize efficiency or benefits while joining to develop their activities (Gero et al. 2005). In this study, up to 94 % of all possible paired associations were non-significant, reaffirming the fluid nature of the community; however, 78 % of the non-random pairs exhibited moderate to high membership (Figure 4). The congregation of prey within a limited area could help explain the random encounters for many of these dolphins (Morteo et al. 2012); on the other hand, the close associations for a small part of the community is likely the result of male alliances, and female bands (sensu Connor et al. 2000), by means of the sexual segregation occurring in the area (Morteo et al. 2014).
We found that dolphin interactions seem to modulate group size (Figure 2), and these are inherently associated to their own activities (Connor et al. 2001; Figure 3 and 5). The positive correlation between the level of association and the diversity of activities (Figure 6) was expected, as the context of the interactions between dyad members should become richer over time (Gero et al. 2005); however, the fact that 17 of the 237 significant dyads involved partnerships with specific purposes suggests that only a handful of these individuals find larger benefits from exploiting the specific abilities of selected partners. Lusseau et al. (2006) evidenced the importance of roles for specific individuals within a social network, where previous knowledge of group members and reduced number of participants helped to coordinate actions and facilitated cooperation; however such interactions were rare and deserve further attention.
Knowledge of the social structure of dolphin communities is important for assessing their ecological and evolutionary patterns; and behavioral data helps to understand how the animals adapt to the habitat and its selective pressures. However, both aspects are rarely combined to determine the nature of their associations, and how these are modified by their life conditions, including natural and anthropogenic factors. Human developments cause that coastal bottlenose dolphins face increasing disruptions within their core areas of distribution, therefore these aspects should be considered while developing and applying conservation and management strategies and policies.
Acknowledgments
This research is part of the lead author's MSc thesis at the Universidad Veracruzana, Mexico, where she obtained fellowship from CONACyT. J. Montano, I. Hernández, V. Del Castillo and N. Medellín were involved in surveys and data collection. Fieldwork was carried with authorization from SEMARNAT permits SGPA/DGVS/00351/06 (E. Morteo) and SGPA/DGVS/00870/07, 02788/07, 01344/08 and 01649/08 (C. Bazúa). This work was supported by the following grants: PROMEP Apoyo a Nuevo PTC (E. Morteo) and CAMyCRA (E. Morteo, and H. Pérez-España), CONACyT grant 45468 (E. Velarde) and the Marine Mammal Laboratory of Universidad Veracruzana and Acuario de Veracruz, A.C.











 nova página do texto(beta)
nova página do texto(beta)