Introduction
It is well known that polymeric membranes are typically used for several water treatment applications, including wastewater streams from agro-food (Castro-Muñoz, Yáñez-Fernández, & Fíla, 2016), textile (Van Der Bruggen, Lejon, & Vandecasteele, 2003), petroleum industry (Alzahrani & Wahab, 2014), and removal of pollutants from drinking water (Kim & Van Der Bruggen, 2010). These membranes generally are aimed to decrease the pollutants in the wastewater (Castro-Muñoz, Barragán-Huerta, Fíla, Denis, & Ruby-Figueroa, 2018; Castro-Muñoz et al., 2016; Van Der Bruggen et al., 2003). At this point, pressure-driven membrane processes, such as microfiltration (MF), ultrafiltration (UF), are considered as alternatives for the removal of large amounts of organic macropollutants; while nanofiltration (NF) and reverse osmosis (RO) have proven their efficacy in withdrawing micropollutants (Castro-Muñoz, Rodríguez-Romero, Yáñez-Fernández, & Fíla, 2017a; Rajesha, Vishaka, Balakrishna, Padaki, & Nazri, 2017).
Over the last decade, numerous studies have been devoted to the manufacture of synthetic membranes for specific applications; displaying acceptable features, such as permeability, selectivity, chemical and physical properties. To date, many organic and inorganic materials have been used in the preparation of membrane; inorganic membranes are generally prepared using materials such as ceramics, metals and glass; while organic membranes are based on polymers or composite materials (Ulbricht, 2006). Particularly, ceramic membranes display higher thermal, chemical and mechanical stability than the polymeric membranes. In addition, the hydrophilicity and surface charge of ceramic membranes are higher than the polymeric ones. Thereby, ceramic membranes can be used under extreme conditions of pH, temperature and high oxidizing environment (Yong, Wahab, Peng, & Hilal, 2013). On the other hand, polymers offer great design flexibility being generally cheaper, e.g., MF and UF membranes based on polysulfone (PSF), polyethersulfone (PES), polyacrylonitrile (PAN), polypropylene (PP), polytetrafluoroethylene (PTFE), and polyvinylidine fluoride (PVDF). Nevertheless, there is a need for enhancing the separation performance of these polymeric membranes, as well as improve some other physic-chemical properties such as stability, hydrophilicity profile and fouling resistance, being the latest the main limiting factor in large-scale applications. Fouling phenomenon generally involves the accumulation of organic-inorganic matter on membrane surface and inside the pores. In this case, the biofouling is the most intrinsically complex form of fouling. Biofouling is a consequence of irreversible microbial cell adhesion (one or several types of microorganisms), followed by colonization on membrane surface forming a microbial biofilm (Flemming, 1997). Once the biofilm is formed at membrane surface, it makes extremely difficult its removal using external agents (Subramani & Hoek, 2008). Moreover, biofilm restricts the solvent permeation across the membrane that leads to increase the transmembrane pressure and thus influencing the separation performance. Therefore, the incorporation of nanomaterials into polymeric membranes can help to mitigate the fouling phenomenon; at the same time, it contributes to enhance other key properties. The following section provides an overview about the improvements of incorporating different nanomaterials into polymeric membranes in the preparation of nanocomposite membranes for water treatment.
The strategy of nanocomposite membranes for water treatment
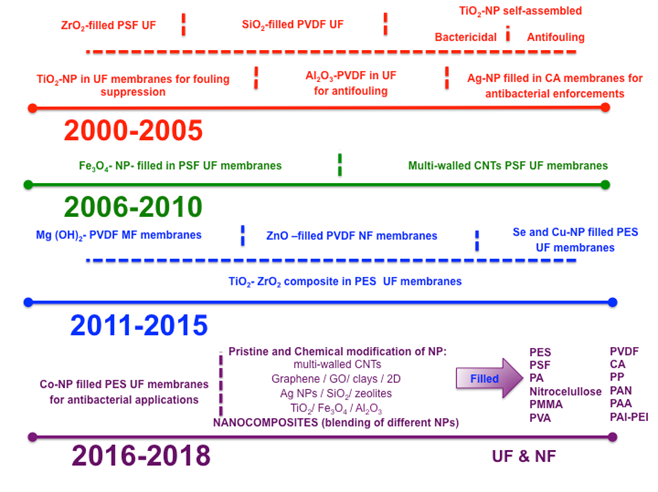
Typically, nanocomposite membranes are prepared by incorporating nanomaterials into a macroscopic polymeric material. The nanomaterials may be either coated onto membrane surface or dispersed in the polymer solution before membrane casting (Castro-Muñoz, Fíla, & Dung, 2017b). In this sense, the dispersed materials, commonly known as fillers, are embedded into the polymeric matrix to generate polymer-nanocomposite membranes which are also referred as mixed matrix membranes (Castro-Muñoz, Martin-Gil, Ahmad, & Fíla, 2017c) or nano-enhanced membranes (Mueller et al., 2012). The nanocomposite membranes are a potential alternative to face several challenges, such as i) improve the performance in terms of permeability and selectivity, ii) enhance the hydrophilicity, which may suppress the accumulation of pollutants and foulants, iii) enhance rejection efficiencies, and iv) improve thermal and mechanical properties. Such enhancements can be reached due to the fact that fillers tend to change the surface properties of the membranes influencing their separation performance. Today, the fabrication of these membranes is one of the current applications of the nanotechnology in membranes for water treatment (Ursino et al., 2018). Figure 1 shows an overview about the progress and advances in the field. For instance, nanomaterials-based membranes have demonstrated low-fouling through adding the inorganic particles (Kim & Van Der Bruggen, 2010). When dealing with the development of nanocomposite membranes, different types of nanomaterials have been proposed such as silver (Ag) (Prince, Bhuvana, Boodhoo, Anbharasi, & Singh, 2014); titanium (TiO2) (Zhang, Shi, & Liu, 2013); zinc (ZnO) (Balta et al., 2012); copper oxide (CuO) (García et al., 2018); carbon nanotubes (CNTs) (Celik, Park, Choi, & Choi, 2011); graphene oxide (GO) (Xia & Ni, 2015); aluminum (Al2O3) (Arsuaga et al., 2013); silicon (SiO2) (Yu et al., 2009); iron (Fe3O4) (Alam et al., 2016); cobalt (Co) (Gzara et al., 2016); zirconium (ZrO2) (Maximous, Nakhla, Wan, & Wong, 2010); clay nanoparticles (Mierzwa, Arieta, Verlage, Carvalho, & Vecitis, 2013), and zeolites (e.g., NaX) (Fathizadeh, Aroujalian, & Raisi, 2011).
Carbon nanotubes (CNTs) have recently attracted the attention of researchers due to their extraordinary electrical, mechanical, thermal properties and partial antibacterial activity (e.g., Pseudomonas aeruginosa), being important the latest property for water purification applications. For instance, CNTs composite membranes have shown significant antimicrobial activity (80-90%) toward Gram-positive and Gram-negative bacteria as well as virus removal (Ahmed, Santos, Mangadlao, Advincula, & Rodrigues, 2013). Thereby, such membranes could be used as membrane filters for drinking water treatment. Furthermore, CNTs can modify the physico-chemical properties of the membranes, which encourage their potentiality for several applications. Typically, the inner pores of CNTs tend to act as selective nanopores, and thus CNT-filled membranes tend to display an enhanced permeability without a decrease in their selectivity, while enhancements in mechanical and thermal properties can be obtained as well.
Carbon nanotubes (CNTs) have recently attracted the attention of researchers due to their extraordinary electrical, mechanical, thermal properties and partial antibacterial activity (e.g., Pseudomonas aeruginosa), being important the latest property for water purification applications. For instance, CNTs composite membranes have shown significant antimicrobial activity (80-90%) toward Gram-positive and Gram-negative bacteria as well as virus removal (Ahmed et al., 2013). Thereby, such membranes could be used as membrane filters for drinking water treatment. Furthermore, CNTs can modify the physico-chemical properties of the membranes, which encourage their potentiality for several applications. Typically, the inner pores of CNTs tend to act as selective nanopores, and thus CNT-filled membranes tend to display an enhanced permeability without a decrease in their selectivity, while enhancements in mechanical and thermal properties can be obtained as well.
Another material, which has been applied as filler in nanocomposite membranes, is titanium dioxide (TiO2). This nanomaterial has good thermal and chemical stability, low human toxicity and photocatalytic properties. In addition, it generally remains unchanged during degradation process of micro-organisms and organic compounds. The material becomes excited under UV irradiation, this energy promotes the electron to the conduction band of TiO2, creating a pair of a negatively charged free electron and a positively charged electron hole. The electrons and holes give strong reducing and oxidizing activities, and subsequently, they can react with atmospheric water and oxygen to yield reactive oxygen species, such as hydroxyl radicals (-OH), superoxide anions (O2 −), and hydrogen peroxide (H2O2) (Liou & Chang, 2012). During irradiation of TiO2 nanomaterials, the hydroxyl radicals and superoxide ions are able to react with most biomolecules, exhibiting bactericidal and virucidal activity.
Silver (Ag)-based materials, such as Ag nanoparticles, Ag salts, metal oxide composites and Ag-impregnated zeolite, tend to offer antimicrobial properties as well. Their activity generally depends on the physicochemical properties of the particles (e.g., size, shape, and chemistry). In particular, Ag nanoparticles reduce the activity of bacteria due to a synergistic effect between direct particle-specific biological effects and the release of Ag+ ions. Furthermore, Ag nanoparticles can stick to the bacterial cells that influence negatively the permeability and respiration of the bacteria, together with a possible cell lysis. Regarding the preparation of nanocomposite membranes using Ag, cellulose acetate (CA), chitosan, polyacrylonitrile (PAN) and polysulfone (PSF) are some of the polymeric materials used as matrix (López-Heras, Theodorou, Leo, Ryan, & Porter, 2015; Sile-Yuksel, Tas, Koseoglu-Imer, & Koyuncu, 2014). Sile-Yuksel et al. (2014) indeed studied the effect Ag nanoparticles filled in different types of polymers (e.g., PES, PSF and CA). The authors reported that Ag nanoparticles were homogeneously located along the membrane matrix in both skin layer and sub-layer but they protruded from the top surfaces of PSF and PES membranes. In a different study, the anti-bacterial properties of these nanoparticles incorporated in chitosan were evaluated using E. coli and Pseudomonas, which generally promote the biofouling by secreting extracellular polysaccharides (Zhu, Bai, Wee, Liu, & Tang, 2010). The authors reported a significantly anti-bacterial performance of these composite membranes. Moreover, the anti-biofouling properties were studied for 10 days, being stable in such period.
Copper (Cu)-based nanomaterials have also demonstrated bactericidal and fungicides activities against viruses and algae (Ren et al., 2009; Varkey & Dlamini, 2012). It is quite possible that Cu materials can interact with the bacteria by producing reactive oxygen species, lipid peroxidation, protein oxidation and DNA decomposition, leading to generate superoxide anions (Tamayo, Azócar, Kogan, Riveros, & Páez, 2016). In addition, Cu2+ ions may react with phosphorus or -SH groups presented in biomolecules (e.g., DNA and proteins); they can act by disturbing biochemical processes, leading the protein denaturation (Ruparelia, Chatterjee, Duttagupta, & Mukherji, 2008). Xu, Feng, Chen and Gao (2012), and Xu et al. (2015) confirmed the antibacterial properties (against E. coli, efficiency 71.5%) of Cu nanoparticles filled in PAN membranes; while the permeability of the composite membranes was enhanced in comparison with the pristine PAN membranes. Particularly, Xu et al. (2015) reported that using cross-linked PAN-filled Cu(II) membranes were able to modulate the release of Cu2+, which can provide antibacterial efficiency up to 95%. It is important to mention that the biofilms formation was suppressed during 6-months testing.
Zinc oxide (ZnO) is multifunctional inorganic nanomaterial which is also interesting due to its physical and chemical properties, e.g., catalytic, antibacterial and bactericide activities. This nanomaterial is able to absorb hydrophilic hydroxyl groups (-OH), its surface area is relatively higher than other inorganic materials (Shen et al., 2012). Regarding its incorporation for producing nanocomposite membranes, ZnO tends to improve specific properties in polymers, including the hydrophilicity, mechanical and chemical properties (Lin et al., 2009). The incorporation of ZnO also generates improvement on hydrophilicity of PES NF membranes; this results in higher permeabilities in ZnO-filled nanocomposite membranes. Also, fouling resistance during the filtration of solutions containing humic acid has been reported (Balta et al., 2012). On the other hand, different nanomaterial, like graphene oxide (GO), is a carbon-based material produced by oxidizing of the graphene. GO exhibits a hydrophilic nature. This nanomaterial is also able to improve mechanical and thermal properties of polymeric membranes (Ionita, Pandele, Crica, & Pilan, 2014). In principle, GO possesses functional groups which provide the possibility of carrying out several surface-modification reactions, e.g., carrying various hydrophilic functional groups (-NH2, -OH, -SO3H) (Enotiadis, Angjeli, Baldino, & Nicotera, 2012; Liu et al., 2017). The GO has recently considered in the preparation of nanocomposite membranes for water treatment, such as water desalination, removal of toxic ions and organic molecules in polluted water ( An,Yang, Wang, & Liu, 2016). In fact, GO is potentially considered as one of the promising nanomaterials applied for the removal of pharmaceutical traces from water and wastewater (Sophia, Lima, Allaudeen, & Rajan, 2016). Chang et al. (2014) analysed the synergistic effect of GO and polyvinylpyrrolidone (PVP) on the performance of PVDF membrane. Certainly, it has demonstrated that the membrane hydrophilicity and the anti-fouling properties were enhanced by incorporating GO into PVP. The authors reported that the improvement is attributed to the formation of hydrogen bonds between PVP and GO.
Nowadays, the use of nanomaterials in composite membranes is a current approach in the field, making to researchers to propose new inorganic nano-sized materials, for example, MCM-41 silica, SiO2 (Yin, Kim, Yang, & Deng, 2012), zeolite MCM-22 (Wang, Li, & Xu, 2006), clays (Mierzwa et al., 2013), alumina (Al2O3) and Fe3O4. All these nanomaterials have started to be implemented in membranes pursuing the water purification and desalination, or wastewater treatment. This is due to specific properties based on their structures. For example, zeolites are crystalline alumina-silicate materials, having three-dimensional framework structures. Similarly, they tend to enhance hydrophilicity in the nanocomposite membranes, this also leads enhancements in permeability and better anti-fouling properties; however, zeolites also possess a molecular-sieving separation mechanism, which can contribute to better separation efficiencies.
General remarks
To date, the incorporation of different classes of nanomaterials (e.g., ZnO, Ag or Cu-based materials, GO, TiO2, Al2O3, Fe3O4, zeolite, clay, SiO2, graphene oxide) into polymeric membranes tends to enhance the hydrophilicity depending on the type of polymer, contributing to suppress the fouling phenomenon in water treatment. Additionally, the filler materials can also provide the possibility to improve some other properties (e.g., mechanical, thermal, and chemical) as well.
These current findings provide valid inputs concerning the potentialities of these smart membranes in water purification, according to the antibacterial properties of the fillers. Particularly, the exploitation of composite membranes can be synergistic towards efficient water treatment (e.g., wastewater processing), if there is a coupling to other technologies, e.g., photocatalytic process (Zhao, Chen, Quan, Yu, & Zhao, 2016), electrocoagulation, electrofiltration (Yang, Chen, Yang, & Yen, 2016), or membrane bioreactor (Khalid, Abdel-Karim, Ali-Atieh, Javed, & McKay, 2018). Finally, it is important to take into account that the compatibility between the nanomaterial and polymer is crucial in order to synthetize highly efficient nanocomposite membranes.











 nueva página del texto (beta)
nueva página del texto (beta)