Introduction
Coking wastewater is the wastewater produced in the process of recovery of coal coking product. It is usually known to contain nitrogen heterocyclic compounds (NHCs) and polycyclic aromatic hydrocarbons (PAHs) that are difficult to biodegrade. It also contains a variety of other toxic substances that act as inhibitors of microbial activity. Therefore, the traditional biochemical treatment technology is difficult to meet the national emission standards for the removal effect of biological nitrogen is not obvious. NHCs account for 20-30% of the total organic components and are the main component of organic pollutants in coking wastewater (Joshi et al., 2015). The substitution of N and S makes these heterocyclic organic compounds more toxic than the corresponding non-heterocyclic organic compounds (Eisentraeger et al., 2010). It is, therefore, crucial to study the biodegradation of nitrogen heterocyclic compounds in coking wastewater in the coexistence of various other organic and inorganic pollutants.
In coking wastewater, quinoline (Q), indole (I), pyridine (Pd), phenol (P) and ammonia nitrogen (N) constitute a co-substrate system. Addition of a contaminant in the co-substrate system can either promote or inhibit the degradation of another one (Rasool et al., 2015; Martinez et al., 2015; Wen et al., 2011). Phomopsis liquidambari, Brevundimonas sp. and Shinella zoogloeoides have been identified to possess strong specificity for degrading indole, quinoline and pyridine, but not towards coking wastewater with a complex composition (Chen et al., 2013; Wang et al., 2015; Bai et al., 2009). On the other hand, many studies have shown that white-rot fungi have a non-specific nature towards the degradation of the substrate and direct or indirect degradation ability for most aromatic compounds. Furthermore, white-rot fungi were also found to degrade a large number of single or mixed matrix contaminants (Pointing et al., 2001). The ability of degradation of multiple single substrate pollutants by white-rot fungi had also been demonstrated in some recently conducted studies (Ren et al., 2012; Stella et al., 2016). On the basis of these revelations, the degradation behavior and kinetic characteristics of four co-substrate systems in simulated coking wastewater were studied in this paper. Further study of the feasibility of degradation of nitrogenous heterocyclic compounds by white-rot fungi in the effect of metal ions in actual coking wastewater was also studied.
Materials and Methods
Reagent
Pyridine, quinoline and indole (AR, Sinopharm Group Chemical Reagent Co., Ltd., China); 2,2'-nitro-bis (3-ethylbenzothiazole-6-sulfonic acid) (ABTS) (Sigma-Aldrich); glacial acetic acid: AR; methanol: chromatographic pure; ammonium tartrate; glucose; ammonium sulfate ((NH4)2SO4); monopotassium phosphate (KH2PO4); magnesium sulfate heptahydrate (MgSO4·7H2O); manganese(II) sulfate monohydrate (MnSO4·H2O); copper(II) sulfate pentahydrate (CuSO4·5H2O) and microporous membrane (0.45μm).
Culture of White-Rot Fungi and Preparation of Medium
White-rot fungal strain (Pleurotus sp.) No. BP, selected and preserved by the Environmental Resources Microbiology Research Laboratory of Huazhong University of Science and Technology, China, was expanded after 4 days as a spare bacterial fluid. The medium used in the experiment was a straw filtrate culture medium, consisting of water (1000 mL), corn stalk powder (30 g), bran (8 g) and cottonseed meal (1.2 g).
Preparation and Testing of Simulated and Actual Wastewater
10 mL bacterial fluid was inoculated into a 250 mL triangle flask containing 100 mL culture medium. A Pd+Q+I co-substrate degradation system was made up by adding certain amounts of pyridine (80 mg/L), quinoline (80 mg/L) and indole (80 mg/L) reserve solutions to the culture flask. The solution thus prepared was then added with phenol (150 mg/L) or ammonia nitrogen (290 mg/L) reserve solutions to simulate the presence of actual coking wastewater. The Erlenmeyer flask, which was inoculated with the bacteria and the substrate, was placed in a shaker (150 r/min) and subjected to a degradation test at 25°С.
The subsequent experimental setups that were carried on actual coking wastewater culture system contained 50mL actual wastewater obtained from Wuhan Iron and Steel Company coking plant (Table 1), and 50 mL of straw filtrate culture medium, which included four degradation systems: no metal system, Mg (II) system, Cu (II) system, Mn (II) system. The concentration of the metal ions in the culture medium was kept 1.0 mM/L and the experimental method followed was similar to that of simulated wastewater based experiment.
Determination
The concentration of quinoline, indole, pyridine and phenol were measured by using Hitachi HPLC (High Performance Liquid Chromatography) (Hitachi pump (L-7100), Hitachi dynamic mixers, UV-vis L-7420 detector and the Hypersil C-18 reverse column (250mm×4.6mmI.D., 5μm)). The standard curve of characteristic absorption peaks of organic compounds was used for conclusion could be got. The conditions for the determination of indole and phenol were as follows: the mobile phase was methanol and water (1% HAc), the volume ratio was 80:20, the flow rate was 0.6 mL/min, the detection wavelength was 270 nm, and the injection volume was 20 μL. The conditions for the determination of quinoline were as follows: the mobile phase was methanol and water (1% HAc), the volume ratio was 50:50, the flow rate was 0.6 mL/min, the detection wavelength was 313 nm, and the injection volume was 20 μL; and the conditions for the determination of pyridine were as follows: the mobile phase was methanol and water (1% HAc), the volume ratio is 70:30, the flow rate was 0.4 mL/min, the detection wavelength was 254 nm, and the injection volume was 20 μL.
The biomass dry weight of white-rot fungi was measured with the help of an electronic balance (FA2004). Ammonia nitrogen was analyzed by WT-1 portable ammonia nitrogen analyzer. The laccase activity levels were estimated using a UV-2550 UV spectrophotometer (SHIMADZU CORPORATION, Japan) by the method of Robert. One enzyme activity unit (U) was defined as the absorbance values of per mL degradable solution per minute increased by 0.001 (Bourbonnais et al., 1992).
Degradation of Simulated Coking Wastewater by White-Rot Fungi
Based on the Pd+Q+I co-substrate degradation system, ammonia nitrogen or phenol was added to the degradation system to form Pd+Q+I, N+Pd+Q+I, P+Pd+Q+I and N+P+Pd+Q+I. This was done to simulate coking wastewater for the purpose of analysis of the degradation behavior of white-rot fungi on various nitrogen heterocyclic compounds, ammonia and phenol.
Removal Effect and Reaction Kinetics
The degradation of quinoline by white-rot fungi in four co-substrate degradation systems is shown in Figure 1. After 15 days, the removal rates of quinoline in the four systems of Pd+Q+I, N+Pd+Q+I, P+Pd+Q+I and N+P+Pd+Q+I were, 66.2%, 62.5%, 70.0% and 68.7%, respectively. It is evident that the degradation rate of quinoline in the four systems was almost consistent.
The degradation data of quinoline in four different co-substrate degradation systems were linearly fitted in the zero-order kinetics equation (Table 2). From Table 2, it can be seen that the quinoline degradation rate constant (k 0 ) value in the four systems was about 3.5 mg/L·d, but with minor variations. Compared with Pd+Q+I, the value of k 0 of P+Pd+Q+I and N+P+Pd+Q+I was slightly increased, while for N+Pd+Q+I it was slightly decreased.
Table 2 Kinetic equations and parameters of quinoline degradation in different systems.
| Co-substrate system | Kinetic equation | k 0 (mg/L·d) | r 2 |
|---|---|---|---|
| Pd+Q+I | C=-3.5901t+71.541 | 3.5901 | 0.9443 |
| N+Pd+Q+I | C=-3.3243t+75.279 | 3.3243 | 0.9687 |
| P+Pd+Q+I | C=-3.7027t+72.633 | 3.7027 | 0.9294 |
| N+P+Pd+Q+I | C=-3.6712t+77.110 | 3.6712 | 0.9705 |
The degradation of indole by white-rot fungi in four co-substrate degradation systems is also shown in Figure 2. The fungi could remove more than 99% of indole by the end of 3 days, and it was also found that the process of indole degradation rate was also consistent, with slight modifications.
The degradation data of indole in four different co-substrate degradation systems was found to fit in the zero-order kinetics equation. Table 3 clearly indicates that the average indole degradation rate constant (k 1 ) was about 26.5. Compared with Pd+Q+I, the degradation rate constants of the other three co-substrate degradation systems decreased slightly, but not obvious.
Table 3 Kinetic equations and parameters of indole degradation in different systems
| Co-substrate system | Kinetic equation | k 1 (mg/L·d) | r 2 |
|---|---|---|---|
| Pd+Q+I | C=-27.1t+71.9 | 27.1 | 0.9211 |
| N+Pd+Q+I | C=-26.2t+71.8 | 26.2 | 0.9360 |
| P+Pd+Q+I | C=-26.0t+75.0 | 26.0 | 0.9758 |
| N+P+Pd+Q+I | C=-27.0t+73.5 | 27.0 | 0.9477 |
The degradation of pyridine by white-rot fungi in four co-substrate degradation systems are shown in Figure 3. After 15 days, the removal rates of pyridine in the four systems of Pd+Q+I, N+Pd+Q+I, P+Pd+Q+I and N+P+Pd+Q+I were 20%, 27%, 27.5%, 32.5%, respectively. It was found that the degradation rates of pyridine in the four degradation systems were affected by the co-substrate.
The degradation data of pyridine in the degradation systems was also found to linearly fit in the zero-order kinetics equation. The data presented in Table 4 shows that the pyridine degradation rate constant (k 2 ) was 1.05 in P+Q+I system. Compared with Pd+Q+I, the degradation rate constants were found to increase in the other three co-substrate degradation systems and the N+P+Pd+Q+I system was the most obvious.
Table 4 Kinetic equations and parameters of pyridine degradation in different systems.
| Co-substrate system | Kinetic equation | k 2 (mg/L·d) | r 2 |
|---|---|---|---|
| Pd+Q+I | C=-1.0500t+78.525 | 1.0500 | 0.9611 |
| N+Pd+Q+I | C=-1.4194t+78.983 | 1.4194 | 0.9789 |
| P+Pd+Q+I | C=-1.4653t+80.275 | 1.4653 | 0.9599 |
| N+P+Pd+Q+I | C=-1.8198t+81.386 | 1.8198 | 0.9755 |
Effect of Co-Substrate on Pd+Q+I System
It could be seen from Figure 4 that the concentration curve of ammonia nitrogen and quinoline were basically the same in the first 3 days of the N+Pd+Q+I system. After 3 days, the indole concentration decreased to zero and the ammonia concentration began to rise. In Figure 5, it is shown that phenol and indole are the carbon sources that can be directly used by the white-rot fungi in the P+Pd+Q+I system. Hence, on the 3rd and 4th day, white-rot fungi removed more than 99% indole and phenol. Figure 6 further illustrates that indole and phenol in the N+P+Pd+Q+I system were completely removed by the fungi on 3rd and 5th day. The concentration of ammonia nitrogen was also found to be the lowest in these two days, 241 mg/L and 246 mg/L, respectively. From these three figures, it is clear that the effect of indole degradation was not obvious when ammonia nitrogen or phenol was used as a co-substrate. In addition, ammonia nitrogen was found to have inhibitory effects on quinoline degradation, but the promoting effects of phenol on quinoline degradation were greater than the inhibition effects of ammonia nitrogen.
Changes in Enzyme Activity, White-Rot Fungi Biomass and pH
As illustrated in Figure 7, on the 3rd day, the laccase activities of white-rot fungi in Pd+Q+I, N+Pd+Q+I, P+Pd+Q+I and N+P+Pd+Q+I reached the maximum levels of 366, 485, 401 and 411 U/mL, respectively. Compared with Pd+Q+I, the other three systems had even higher laccase activity peaks.
As illustrated in Figure 8, the growth rates of the fungi in the Pd+Q+I, N+Pd+Q+I, P+Pd+Q+I and N+P+Pd+Q+I conditions reached the maximum values of 0.106, 0.102, 0.100 and 0.085 g/d on the 2nd, 2nd, 3rd and 2nd days, respectively.
Figure 9 illustrates that the pH of the four systems in the first four days also changed similarly and then again reached the lowest values on the 4-5th day. This drop in pH can be attributed to the degradation of phenol and indole and their intermediates. However, other aromatic acid based compounds present (the degradation products of the cellulosic components of culture media) also play an important role. It was observed that the pH of N+Pd+Q+I and N+P+Pd+Q+I systems after four days was comparatively higher than that of the other two. This may be caused due to the presence of a stable buffer system formed between ammonia nitrogen and aromatic acids after the concentration of ammonia nitrogen was stable, and showed weak alkalinity.
Treatment of Actual Coking Wastewater by White-Rot Fungi
The degradation of actual coking wastewater was mainly dependent upon the role of divalent metal ions in the degradation of nitrogen heterocyclic compounds. In addition, other factors that influenced the process of degradation included the concentration of other major pollutants in coking wastewater and the biological characteristics of white-rot fungi in the four degradation systems (no metal ions (CK) / Mg (II) / Cu (II) / Mn (II)). The reason behind the selection of Cu (II), Mn (II) and Mg (II) in the present study was the fact that these divalent ions exist in the actual coking wastewater and it was intended to elucidate the effects that their changing concentration may imply on the biological properties of white rot fungi and hence on the rate of degradation of the wastewater. Since COD of the substrate in the culture medium was known to be very high, which in turn had a profound influence on the determination of the COD of the system; it was not considered for analysis in the experiment.
Effect of Divalent Metal Ion on the Removal of Nitrogen Heterocyclic Compounds
According to the Figure 10, white-rot fungi were cultured for 15 days on solid medium, and the removal rates of quinoline in the four degradation systems were 65%, 76%, 65% and 78%, respectively. It was found that the addition of Mg (II) and Mn (II) promoted the degradation of quinoline by white-rot fungi and even improved (slightly) the removal rate of quinoline. On the other hand, Cu (II) was not found to have any significant effects on the degradation of quinoline by white-rot fungi. It could be seen from Figure 11 that the removal rate of indole in the system of Mg (II), Cu (II) and Mn (II) could reach more than 99%, while Figure 12 illustrates that the removal rates of pyridine in the four systems were 38%, 32%, 24% and 31%, respectively. It was also found that the divalent metal ions inhibited the degradation of pyridine, out of which the inhibition effect of Cu (II) was the most obvious.
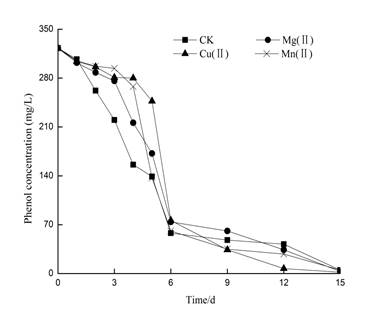
It could be seen from Figure 13 and Figure 14 that the change in ammonia nitrogen concentration also followed a similar trend. The fluctuation range during the 0-6th days was very less and further decreased during the 6-12th days. An increase in fluctuation was observed during the 12-15th days. The changes in ammonia nitrogen concentration were also found to be similar to that of the simulated wastewater, both of which go through the process of declining first followed by a systematic rise in the values. Due to the high concentration of indole and phenol in the simulated wastewater, the overall change in ammonia nitrogen concentration was mainly brought about due to degradation of indole and phenol. In the actual coking wastewater, the change of ammonia concentration is mainly related to the removal of phenol (phenol concentration is 323 mg/L, and indole concentration is small in the actual coking wastewater). Figure 13 clearly demonstrates that phenol was removed significantly on the 6th day after which the ammonia concentration began to decrease. White-rot fungi were found to remove more than 99% of the phenol content in four of these systems. However, the initial degradation of phenol was slow, especially due to the inhibition of laccase secretion of white-rot fungi in the early stages, as the fungi were in its domestication stage. After five days, the fungi adapted to the actual coking wastewater environment, which led to an increase in the laccase enzymatic activity levels that further resulted in the rapid degradation of phenol.
Change in Biomass and Enzyme Activity Levels of White-Rot Fungi
It can be seen from Figure 15, that on the first five days, the laccase secretion of white-rot fungi was inhibited. The laccase activity levels were maintained at about 10 U/mL, which is due to the simultaneous presence of a variety of other toxic and harmful substances in coking wastewater that may play significant roles in inhibition of enzyme secretion in white rot fungi (Asif et al., 2017). After five days of domestication, the white-rot fungi supposedly adapted to the actual coking wastewater culture environment which subsequently led to an increase in the enzyme activity levels. Results obtained from the present experiment demonstrated that the laccase secretion of white-rot fungi in the CK system was similar to Mg (II) system and Mn (II) system. This indicated that the addition of 1.0 mM/L of Mg (II) and Mn (II) had no significant effects on the laccase secretion activities of the fungi. Furthermore, the enzymatic activity of the white-rot fungi in the Cu (II) system was the same as that of the other three systems, especially in the first five days. On the 6-12th day, the activity of laccase was found to increase slowly and the laccase secretion was still inhibited. On the 12-15th day, the activity of laccase was found to increase rapidly and reached the level of 412 U/mL on the 15th day. It was thus concluded that the presence of 1.0 mM/L Cu (II) could promote laccase secretion.
As is shown in Figure 16, the growth rate of white-rot fungi biomass in CK, Mg (II), Cu (II) and Mn (II) in the actual coking wastewater reached a maximum of 0.0166, 0.0238, 0.0111 and 0.0373g/d on 2nd, 1st, 1st and 1st day, respectively. It was thus concluded that the presence of 1.0 mM/L Mg (II) and Mn (II) could promote the growth of white-rot fungi, due to which the fungi maintained a high biomass growth rate. In contrast to the influence on the laccase secretion of white-rot fungi, 1.0 mM/L Cu (II) inhibited the development of white-rot fungi strains, and the growth rate of white-rot fungi biomass was low during the whole experiment. The two peaks of biomass growth rate that were observed during the experiment corresponded to the 1st day and the 6th day in the degradation system.
As that the straw filter medium was diluted twice caused the reduction of nutrient, while the actual coking wastewater composition is complex and toxic, in the actual coking wastewater culture environment, the growth rate of white-rot fungi biomass was found to be much lower than the simulated wastewater.
Discussion
In the simulated wastewater, the addition of ammonia nitrogen and phenol affected the enzyme secretion and enzyme activity of white-rot fungi, especially in the Pd+Q+I degradation system. The addition of ammonia nitrogen provided a nitrogen-rich culture environment to the white-rot fungi, and the phenol structure (with phenolic hydroxyl) induced the laccase secretion of the white-rot fungi. It was also found that quinoline could be better degraded in neutral or acidic environments while the degradation rate declined rapidly in an alkaline environment (Thomsen et al., 1998). The addition of ammonia nitrogen increased the pH of the system and thus inhibited the degradation rate of quinoline. However, the addition of phenol reduced the pH of the system to promote the degradation of quinoline. It was observed that OH plays a significant role in quinoline degradation due to the fact that phenolic hydroxyl groups on phenol are less stable (Camarero et al., 2010). They can thus attack the aromatic compounds, produce positive carbon ions and then induce a series of reactions that promotes the degradation of quinoline. On the other hand, the alcoholic hydroxyl groups on the ammonia nitrogen (ammonium tartrate) are relatively stable and have little effect on the degradation of quinoline.
In the actual coking wastewater, presence of moderate concentrations of Mg (II) and Mn (II) could promote the growth of white-rot fungi, so that more laccase was secreted and the removal rate of quinoline was slightly improved (Baldrian, 2003). The rate of initiation of the laccase secretion of white-rot fungi in the actual coking wastewater environment was much slower than simulated wastewater. Laccase activity was maintained at a low level on 0-12 days, but this is not due to the fact that some metal ions in the solution causes fungi in oxidative stress (Galhaup et al., 2001). The lower enzyme activity is mainly due to the complex composition of the actual coking wastewater. Such wastewater contains a lot of toxic and harmful substances which inhibit secretion of laccase (Asif et al., 2017). On 12-15th day, the laccase activity in the actual coking wastewater of Cu (II) system was twice as much as the laccase activity in other systems. It has already been established that the activity center of white-rot fungi laccase constitutes of four Cu ions constitute (Strong et al., 2011; Baldrian et al., 2006; Giardina et al., 2010). White-rot fungi allow direct passage of Cu (II) through its cell walls. Hence, the presence of Cu ions, even in concentrations of less than 1 mM/L can also act as an inducer and promote laccase secretion. Meanwhile, the presence of a variety of other aromatic and phenolic compounds induced continued maintenance of an activated state of the white-rot fungi which further promoted its laccase secretion activities (Tychanowicz et al., 2006). Cu (II) can also directly activate the active site of extracellular laccase enzymes to improve its activity.
Conclusion
In simulated wastewater, ammonia nitrogen had an inhibitory effect on quinoline degradation, while the promoting effects of phenol on the same were even greater than the inhibition effect of ammonia nitrogen. White-rot fungi could remove more than 99% of indole only after three days. On the other hand, ammonia nitrogen and phenol had little effect on the degradation of indole, but they were beneficial for the degradation of pyridine and improving the peak of laccase activity. In actual coking wastewater, Mg (II) and Mn (II) promoted the degradation of quinoline and slightly improved the removal rate of quinoline. However, Cu (II) had no effect on the degradation of quinoline. It was concluded that the divalent metal ions had no significant effects on the degradation of indole, but they had a profound impact on the inhibition of the degradation of pyridine. Among all the metals studies, and the inhibition effect of Cu (II) was the most obvious. It is also important to note that though the addition of 1.0 mM/L Mg (II) and Mn (II) had no significant effects on the secretion of laccase, they could still promote the growth of white-rot fungi so that the fungi maintained high biomass growth rate. Conversely, 1.0 mM/L Cu (II) could promote the secretion of laccase, but inhibited the development of white-rot fungi.











 text new page (beta)
text new page (beta)