Introduction
Macrobenthic invertebrates play important roles in the ecosystem functions of lakes through geochemical cycling of elements, including nutrients that limit overall productivity (Holker et al., 2016). Macrobenthic animals, spending all or most of their life on the bottom of a water body, exhibit a weak ability to migrate, making them good indicators of the ecological quality of aquatic environments worldwide (Wong et al., 2015; Dauer, 1993; Chainho et al., 2006). Furthermore, they have been employed to monitor changes in environmental variables over decades (Resh, 2007). Because benthic invertebrates exhibit different abilities to adapt to changes in environmental conditions, fluctuations in their relative abundances can provide insights into the causes and severity of pollution (Wallace et al., 2015; Beck and Hatch, 2009; Richman and Somers, 2010; Marzin et al., 2012). Structural measures of macrobenthic assemblages, such as their community structures and diversity, can be used as integrative indicators of environmental quality. Macroinvertebrate communities are an important component in nutrient cycling and pollutant transformation (Signa et al., 2015; Lawrence et al., 2016). Because environmental pollution is harmful to native taxa and often encourages the intrusion of invasive taxa, human activity may affect macrobenthic communities (Gichana et al., 2015; Galil, 2000). Changes in environmental parameters (e.g., salinity, sedimentation and eutrophication) might also play a crucial role in these adverse effects (Brock et al., 2016; Nishijima et al., 2015; Lamptey and Armah, 2008; Yoon et al., 2017; Pereira et al., 2012; Mandal and Harkantra, 2013). Studies have demonstrated that seasonally varying conditions in terms of sediment parameters, such as pH, salinity and flow rate, are significant determiners influencing the distribution of macrobenthic communities in estuaries (Courtney and Clements, 1998; Kilgour et al., 2008; Feebarani et al., 2016; O'Brien and Keough, 2013). However, previous studies have mainly focused on estuaries, lagoons and marine areas (Marques et al., 2013; Romeo et al., 2015; Jordana et al., 2015; Gillett et al., 2015), and the relationships between macrobenthic indicators (structure and diversity) and environments have rarely been investigated in shallow freshwater lowland lakes (Cai et al., 2017; Li et al., 2016).
Shallow lowland lakes are critical components of the water cycle and provide essential service functions, including storing and purifying water, providing habitats for animals, protecting biodiversity, and irrigation. Because the water capacity is low and sediment interface processes are weak in shallow lakes, such lakes are seriously disturbed by bioenvironmental stressors, especially in lowland regions (Havens et al., 2007). Water quality deterioration leads to eutrophication in shallow lowland lakes. Consequently, shallow lowland lakes face a number of ecological problems, such as habitat destruction, the presence of invasive taxa, and reduction in the functional diversity of communities (Zhang et al., 2016; Rahel, 2002; Angeler and Johnson, 2013). Therefore, to better manage lake ecosystems, researchers and managers need to improve their understanding of the main factors that influenced macrobenthic community diversity in lake ecosystems. Ge Lake (Ch: Ge), the largest lake in southern Jiangsu province in eastern China, is a representative shallow lowland lake. As an important component of the lakes in the vicinity of Tai Lake (Ch: Tai), Ge Lake gives play to a significant role of protecting ecological environments in Tai Lake. Ge Lake is used for commercial navigation, excursions, irrigation and aquaculture (Huang, 2001). Over the past three decades, Ge Lake has suffered anthropogenic pollution from industrial and domestic wastewater and agricultural runoff. The water quality in Ge Lake has been characterized by a decreasing ability to process and assimilate this waste. The proportions of plants in the community have changed, with a reduction in the absolute and relative numbers of aquatic macrophytes. Due to eutrophication and phytoplankton blooms in Ge Lake, the relative proportions of animals in communities, including benthic invertebrates, have been reduced (Guo, 2007). Monitoring of the status and trends of both abiotic and biotic indicators of environmental quality in Ge Lake is currently focused on total nitrogen (TN), total phosphorus (TP) (Xu et al., 2013), algal blooms (Tao et al., 2011; Duan et al., 2012) and technologies to control nutrients and biomass from blooms of a few dominant phytoplankton taxa (Zhang et al., 2011). However, ecological indicators of the aquatic benthic community, such as diversity and evenness have been seldom studied (Wang et al., 2012). Additionally, the relationship between cyanobacterial microcystins (MCs) and macrobenthic communities has rarely been researched in freshwater lakes (Li et al., 2016; Lance et al., 2010).
In this study, structure and diversity of macrobenthic communities as well as environmental variables in Ge Lake and catchments of its upstream tributaries were investigated to characterize the status of the benthic invertebrate communities, to establish a benchmark against which future changes can be interpreted, to ascertain the impacts of water environmental variables on ecological indicators, to distinguish factors influencing structure and diversity of macrobenthic communities, and to offer basic information for lake resource utilization and ecological protection in the future.
Materials and Methods
Study Area
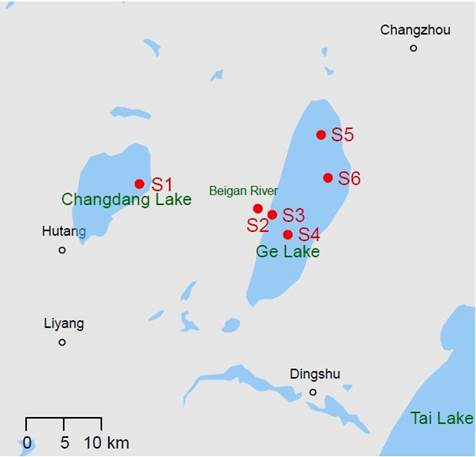
Ge Lake (119°44′15″ to 119°52′56″E and 31°43′04″ to 31°28′19″N) is located at the southwest of Changzhou City, northeast of Tai Lake, and east of Changdang Lake, is situated in the upstream portion of the Tai Lake catchment. Ge Lake is 25 km long and 6.6 km wide, and it has a surface area of 164 km2. The near-shore water depth in the lake is approximately 0.8 to 1.0 m, with an annual average water level of 3.27 m. However, the water level in Ge Lake has decreased due to excessive lake water use for human development since the 1990s. Water flows from Ge Lake into Zhushan Bay in Tai Lake through the Taige Canal.
There were six sampling sites in the study area (Fig 1): site S1 was located at the outlet of Changdang Lake; site S2 was located downstream of S1 on the Beigan River; sites S3 and S4 were located south of Ge Lake; site S5 was located north of Ge Lake, and site S6 flowed into Tai Lake. Sites S1 and S2 were upstream of Ge Lake, while sites S3, S4, S5, and S6 were in Ge Lake. Based on the study (Liu et al., 2005), the difference in macrobenthic community structure between the north and south of Ge Lake was insignificant. Thus, sites S4 and S5 were chosen.
Map of Ge Lake in eastern China and its surroundings. The sampling sites are labeled S1, S2, S3, S4, S5 and S6. Site S1 is located at the outlet of Changdang Lake and sites S3 and S6 are national control sites. According to a previous study (Liu et al., 2005), the difference in macrobenthic community structure between the north and south of Ge Lake was insignificant. Thus, sites S4 and S5 were chosen.
Sampling
Macroinvertebrate Sampling. A Peterson grab (0.0625 m2 sample area) was used to collect sediment samples, including benthic invertebrates. We collected substrate sludge samples in triplicate for the identification and enumeration of macroinvertebrates at each site during each sampling period. According to standard methods (State Environmental Protection Administration of China, 2002), each sediment sample was seriously filtered in situ, and the remainders were kept in 10% formalin and brought to the lab as soon as possible.
Water Sampling. Water samples were synchronously collected with microbenthic samples at the six sites during both May and September between 2008 and 2012. Water samples were collected 0.5 m below the surface into glass bottles for measurements of environmental variables at each site and each time point. YSI multi-water quality parameters were used to analyze water temperature (WT), pH, dissolved oxygen (DO), and conductivity in situ. A Secchi disk was used to examine water transparency (SD). To examine ammonia nitrogen (NH4 +-N), the permanganate index (CODMn), Chlorophyll a (Chl a), total phosphorus (TP), total nitrogen (TN), and MC-LR, water were sampled and stored at 0-4 °C in the dark; and were then processed as soon as possible in the lab.
Analytical Procedures
Macrobenthic Communities. Macrobenthic community samples were washed, sorted out large taxon, appraised to the minimum groups, and recorded the information. Abundances were expressed as individuals m-2. The numbers of taxa (S), Shannon’s diversity index (H’), Margalef’s richness index (D), and Pielou’s evenness index (J) were analyzed using PRIMER Version 6.1.10 for each sample.
Environmental Variables. The concentrations of NH4 +-N, TP, TN, CODMn, and Chl a in water samples were analyzed based on standard methods in the laboratory (State Environmental Protection Administration of China, 2002). Based on previous studies (Rivasseau et al., 1999; Jarkko et al., 2002), MC-LR was analyzed via enzyme-linked immunosorbent assays (ELISAs).
The synthesized trophic state index (STSI) was calculated as an indicator of the magnitude of eutrophication for each sample of water (Equation 1), based on SD, TP, TN, Chl a, and CODMn:
where m is the number of observations; Wj is entropy weight, and TSIj is the trophic state index for variable j. The weight coefficient of each variable was calculated based on previously described methods (Zadeh, 1965; Mon et al., 1994; Qiu, 2001). The correlations between the synthesized trophic state index and lake water environmental quality measures are shown in Table 1.
Table 1 Correlations of the Synthesized Trophic State Index and Water Environmental Quality Classifications in Lakes (Zadeh, 1965; Mon et al., 1994; Qiu, 2001).
| Trophic state | STSI | Water quality |
|---|---|---|
| Oligotrophication | 0<STSI≤30 | Excellence |
| Mesotrophication | 30<STSI≤50 | Good |
| Eutrophication | 50<STSI≤60 | Polluted |
| Supereutrophication | 60<STSI≤70 | Superpolluted |
| Hypereutrophication | 70<STSI≤100 | Hyperpolluted |
STSI: synthesized trophic state index.
Data Analysis
Repeated measures using SPSS 19.0 was employed to test differences between macrobenthic community abundance among years. The spatial difference in the benthic community was analyzed using multivariate statistical analysis. According to the Bray-Curtis similarity matrix, macrobenthic community compositions were compared using a clustering analysis method. Taxon abundance data were log(x+1) transformed. Analysis of similarity (ANOSIM) was used to analyze the differences in macrobenthic community assemblages among sites were analyzed using (Clarke, 1993). According to the Bay-Curtis similarity matrix, non-metric multidimensional scaling (NMDS) was carried out; and data of macroinvertebrate abundance were transformed using log(x+1). PRIMER Version 6.1.10 was used to perform these analyses.
A pairwise Pearson correlation analysis was used to analyze the correlations between environmental variables and macrobenthic indicators (S, H’, D, and J) were analyzed using (Pfeifer et al., 1998; Peeters et al., 2004; Hosmani, 2012). A p-value of < 0.05 was a statistical importance threshold. SPSS 19.0 was used to perform these analyses.
The effect of environmental variables on macrobenthic communities was analyzed using canonical correspondence analysis (CCA). All environmental variables, as well as the T-RF peak area data, were log(x+1)-transformed. Based on 499 permutations under the reduced model and using the αvalue of 0.05, forward selection of the Monte Carlo test was used to discern the environmental variables, with. These analyses were conducted using the software CANOCO 4.5.
Environmental variables with significant multicollinearity (with variance inflation factor >10 and Pearson correlation coefficient |r| >= 0.75) were excluded. Due to their greater correlations (r=-0.761, r=0.790) with SD and CODMn, STSI was removed (Table S2) and the other 11 environmental variables were included in the following analyses. Based on Gaussian error distribution (McGullagh and Nelder, 1989), Generalized linear models (GLMs) were used to analyze the factors of macrobenthic community diversity indices (S, H’, D and J). According to Akaike’s information criterion (AIC) (Akaike, 1974), the best approximating model was selected. GLMs were performed with all environmental variables, except for STSI. R software version 3.3.3 was used to perform all these analyses.
Results
Macrobenthic Assemblage Composition
42 macrobenthic taxa were investigated in this study area during the survey, including 22 arthropods (1 gomphidae, 1 libellulidae, 3 crustaceans and 17 chironomids), 11 molluscs (4 bivalves and 7 gastropods), 7 oligochaetes, and 2 other taxa. Among the macrobenthic assemblages, the oligochaete worm Limnodrilus hoffmeisteri was observed most frequently (96.7%), followed by the gastropod Bellamya purificata, which occurred in 58.3% of samples. Site S2 exhibited the greatest abundance among macrobenthic communities, ranging from 256 to 8256 individuals m-2; site S1 was second. Relatively low abundances were recorded at four sites in Ge Lake, S3, S4, S5, and S6, which ranged from 32 to 1728 individuals m-2. There were 31 taxa of benthic macroinvertebrates observed in Ge Lake (see Table S1 in the SM), including 7 oligochaetes, 7 Mollusca, 14 chironomids, and 3 other taxa, while there were 37 macrobenthic taxa upstream of the lake. In most cases, L. hoffmeisteri exhibited the greatest density, presenting values as high as 1728 individuals m-2 among the macrobenthos.
Multivariate Analyses of Macrobenthic Invertebrates
Based on Bray-Curtis similarity coefficients (at the similarity level of 60%), spatial differences in microbenthic community structure between Ge Lake (sites S3, S4, S5, and S6) and upstream locations (sites S1 and S2) were significant using the non-metric multidimensional scaling (NMDS) analysis (Fig 2). Significant differences in the structure of macrobenthic communities between sampling sites in Ge Lake and upstream areas (R=0.335, p=0.001) were found by one-way ANOSIM. According to the SIMPER procedure, L. hoffmeisteri, B. purificata, the gastropod Bellamya aeruginosa, and the oligochaete Branchiura sowerbyi were the most common taxa at sites S1 and S2, upstream of Ge Lake, while L. hoffmeisteri, B. sowerbyi, and the chironomid Tanypus punctipennis were the primary contributing taxa at the four sites (S3, S4, S5, and S6) in Ge Lake.
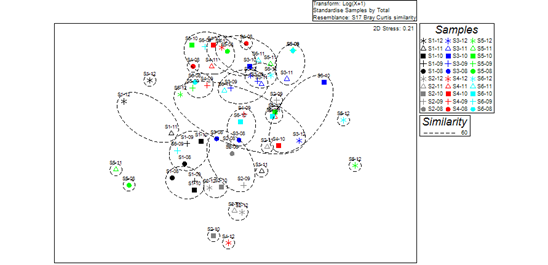
Figure 2 According to Bray-Curtis Similarity Coefficients, Non-metric Multidimensional Scaling (NMDS) among Macroinvertebrate Communities. Year labels: 8 (2008); 9 (2009); 10 (2010); 11 (2011); and 12 (2012).
Compared with the two upstream sampling sites, the four Ge Lake sites exhibited lower macrobenthic community diversity (Fig S1. in the SM). However, the mean Shannon-Wiener diversity index (H’) of the macrobenthic communities in Ge Lake increased between 2008 and 2012, with the exception of 2009 (Fig S2. in the SM).
Environmental Variables
The environmental variables measured at the six sampling sites for the entire sampling period were shown in Table 2. There were statistically significant differences between the four sites in Ge Lake and its two upstream sites. The concentrations of pollutants (NH4 +-N, CODMn and MC-LR) and nutrients (TP and TN) in Ge Lake were greater than in more upstream regions. Based on the correlations between STSI and lake water quality indicators (Table 1), Ge Lake was a supereutrophic lake, while the upstream area was a eutrophic lake.
Table 2 Summary of the Averaged Values from 5 Years of Field Measurements: n=10 Water Environmental Parameters at Ge Lake Sampling Sites.
| Site | S1 | S2 | S3 | S4 | S5 | S6 | p |
|---|---|---|---|---|---|---|---|
| WT (°C) | 22.6±2.5 | 23.3±4.0 | 22.4±3.4 | 22.2±3.4 | 22.4±3.3 | 21.5±3.6 | n.s. |
| pH value | 8.96±0.40 | 8.30±0.53 | 7.86±0.84 | 7.96±0.89 | 7.96±0.79 | 7.80±0.76 | n.s. |
| DO (mg/L) | 8.65±1.49 | 7.90±1.21 | 6.62±1.28 | 6.69±1.31 | 6.64±1.19 | 6.57±1.21 | ** |
| Conductivity (μs/cm) | 620±54 | 605±39 | 672±92 | 643±100 | 660±104 | 665±89 | n.s. |
| NH4 +-N(mg/L) | 0.46±0.12 | 0.43±0.07 | 0.67±0.17 | 0.62±0.16 | 0.69±0.17 | 0.70±0.19 | ** |
| Chl a (mg/m3) | 77.0±44.8 | 45.4±18.1 | 53.6±28.4 | 60.0±38.5 | 61.0±31.2 | 48.9±32.8 | n.s. |
| SD (m) | 0.40±0.06 | 0.48±0.05 | 0.26±0.08 | 0.26±0.09 | 0.30±0.13 | 0.26±0.07 | *** |
| CODMn (mg/L) | 4.30±0.16 | 3.99±0.18 | 6.44±1.59 | 6.36±1.37 | 5.53±1.28 | 6.00±1.48 | *** |
| TP (mg/L) | 0.070±0.009 | 0.059±0.010 | 0.188±0.091 | 0.141±0.033 | 0.148±0.072 | 0.116±0.049 | *** |
| TN (mg/L) | 1.844±0.763 | 1.484±0.467 | 2.731±1.065 | 2.685±1.080 | 2.670±0.996 | 2.705±0.929 | ** |
| STSI | 57.4±5.4 | 56.4±2.1 | 66.2±3.1 | 65.7±4.0 | 64.6±4.4 | 63.7±4.2 | *** |
| MC-LR (μg/L) | 0.110±0.104 | 0.087±0.056 | 0274±0.126 | 0.256±0.142 | 0.243±0.106 | 0.224±0.112 | ** |
| Trophic status | Eutrophication | Eutrophication | Supereutrophication | Supereutrophication | Supereutrophication | Supereutrophication | / |
| Water Quality | Polluted | Polluted | Superpolluted | Superpolluted | Superpolluted | Superpolluted | / |
*: P<0.05; **: P<0.005; ***: P<0.0005; n.s.: not significant.
WT: water temperature, DO: dissolved oxygen, Chl a: Chlorophyll a, CODMn: permanganate index, TP: total phosphorus, TN: total nitrogen, STSI: synthesized trophic state index.
Factors That Influenced Macrobenthic Community Structure and Diversity
According to pairwise Pearson correlation analyses, the relationships between environmental variables and macrobenthic community diversity (S, H’, D and J) varied (Table 3). WT, DO and pH were significantly positively correlated with macrobenthic indicators (S, H’ and D) (p < 0.05). H’ and the Pielou Index (J) were significantly positively correlated with transparency (SD) (R=0.381, p < 0.05; R=0.699, p < 0.05). Conversely, CODMn, STSI and MC-LR were negatively correlated with macrobenthic community diversity indices (S, H’, D and J) (p < 0.05). Meanwhile, H’, D and J were negatively related to TN (p < 0.05).
Based on the CCA analysis, WT, DO, conductivity, NH4 +-N, transparency and TP were the significant environmental variables that explained the macrobenthic community structure variance (Fig 3). Relationships between environmental variables and macrobenthic community diversity indices were analyzed using generalized linear models (GLMs). WT, conductivity, NH4 +-N, Chl a, CODMn, TP and TN were the main factors that influenced macrobenthic community diversity indices (Table 4).
Table 3 Pearson Correlation Coefficients between Macrobenthic Indicators and Environmental Variables (n=60).
| S | H’ | D | J | |
|---|---|---|---|---|
| WT | 0.321* | 0.325* | 0.411** | 0.117 |
| pH | 0.362** | 0.329* | 0.463** | 0.340** |
| DO | 0.509** | 0.298* | 0.304* | 0.478** |
| Conductivity | 0.389* | 0.344* | 0.519** | 0.227 |
| NH4 +-N | -0.024 | 0.125 | -0.166 | 0.134 |
| Chl a | -0.086 | 0.006 | 0.042 | -0.227 |
| SD | 0.112 | 0.381* | 0.240 | 0.699** |
| CODMn | -0.501** | -0.398** | -0.502** | -0.560** |
| TP | -0.098 | -0.240 | -0.175 | -0.373* |
| TN | -0.167 | -0.380* | -0.400** | -0.373* |
| STSI | -0.350* | -0.431** | -0.409** | -0.691** |
| MC-LR | -0.414** | -0.497** | -0.378** | -0.542** |
*: P<0.05; **: P<0.01.
S: Number of taxa, H’: Shannon-wiener diversity index, D: Margalef’s richness, J: Pielou’s evenness index.

Figure 3 Canonical correspondence analysis (CCA) for Macrobenthic Communities and Environmental variable.
Table 4 Results of the generalized linear model (GLM). Selected variables, t values and AIC are given.
| S | Variables | Intercept | DO | NH4 +-N | CODMn | ||
| t value | 2.410* | -1.534 | -1.653 | -3.302** | |||
| AIC | 254.44 | ||||||
| H' | Variables | Intercept | WT | TN | |||
| t value | 0.824 | 2.479* | -2.996** | ||||
| AIC | 78.192 | ||||||
| D | Variables | Intercept | pH | DO | Conductivity | Chl a | CODMn |
| t value | -1.554 | 3.263** | -1.403 | 1.744 | 1.632 | -4.016*** | |
| AIC | 32.682 | ||||||
| J | Variables | Intercept | Conductivity | NH4 +-N | Chl a | TP | |
| t value | 2.442* | 2.680** | 2.412** | 0.525* | -2.680** | ||
| AIC | 4.4466 |
*: P<0.05; **: P<0.01; ***: P <0.001.
Discussion
Changes in Ge Lake Macrobenthic Community
The macroinvertebrate assemblages in Ge Lake have changed significantly over recent decades, and the abundance of more sensitive taxa that require cleaner water has decreased. In general, the macrobenthic community structure is fairly stable, except when environmental variables change dramatically. Between 1991 and 1994, there were 47 macrobenthic animal taxa in Ge Lake, with Ephemeroptera, dragonflies and other sensitive taxa, such as molluscs, which are used as indicators of clean water, being observed in Ge Lake (Zhu et al., 1997). At that time, Ge Lake contained more aquatic macrophytes, with submerged plants covering 87.5% of the area. In addition, there were few fish net-pen cultures in Ge Lake during the 1990s (Zhu et al., 1997; Tao et al., 2010). Compared with the concentrations surveyed in this study (Table 2), the concentrations of TP (< 0.05 mg/L), CODMn (< 3.7 mg/L), and TN (< 1.0 mg/L) (Li and Song, 2013) were very low. During that time, agriculture and fisheries were the main human activity in the area of the Tai River Basin. Between 2002 and 2003, thirty-one taxa of benthic invertebrates were observed, but the number of taxa has decreased significantly. More sensitive taxa have decreased, while more tolerant taxa associated with polluted water have increased (Liu et al., 2005). The species number of mollusk decreased from 24 to 14, while the numbers of intolerant taxa aquatic insects, such as Ephemeroptera, Plecoptera, and Trichoptera (EPT), have decreased from 10 to 3, and the number of oligochaete taxa increased from 2 to 6 between 1995 and 2003. Between 2008 and 2012, there were thirty-one benthic invertebrates recorded, with L. hoffmeisteri being the predominant species in Ge Lake during the survey. Sensitive taxa that inhabit clean water and were observed in the 1990s, such as dragonflies, were rarely found in this study, with only one sensitive clean water taxon, Sinictinogomphus sp. of the Gomphidae family, being observed during the present study. Findings from this study and those of previous studies suggest that macrobenthic community structure and diversity in Ge Lake have been changed by deteriorating environmental conditions; activities associated with human population growth in the catchment of Ge Lake are the likely cause. With the rapid development of the regional population and associated economic activities, there have been greater amounts of pollutants flowing into Ge Lake since the 1990s (Li and Song, 2013). These environmental conditions have promoted phytoplankton growth, limited the absolute and relative numbers of taxa in macrobenthic communities, and promoted a large population increase of L. hoffmeisteri. Results from previous studies (Beghelli et al., 2012; Li et al., 2016; Zhou et al., 2014) have confirmed that oligochaetes such as L. hoffmeisteri and chironomids are widely recognized as indicators of eutrophic conditions in the Tai River Basin.
Diverse habitats are provided by macrophytes, where macrobenthic animals can survive and escape predation. Tews et al. (2004) found that it was necessary to protect macrophytes to protect benthic animal and fish biodiversity. The populations of submerged macrophytes in Ge Lake have rapidly deteriorated. Tao et al. (2010) found that the area covered by submerged plants has declined at an annual rate of more than 10% since 1995. In addition, benthic macroinvertebrates living among aquatic macrophytes were unable to survive (Kennedy et al., 2017; Beresford and Jones, 2010). The lack of plant cover could be the reason for the low number of gastropods found in this study. Previous studies have demonstrated that habitats characterized by gastropods occur in macrophyte-dominated regions (Hu et al., 2016; Jaschinski et al., 2011). In addition, fish net-pen cultures in Ge Lake could likely have contributed to the loss of macrobenthic community biodiversity due to loading of nutrients and habitat destruction, resulting in eutrophication and organic matter loading that has enriched sediments and resulted in decreased dissolved oxygen. Scalable fish net-pen cultures in Ge Lake had occurred since 1995 (Liu et al., 2005). Consequently, waste derived from the feed, including uneaten feed and fish excreta, was released into the water (Jones, 2010; Boaventura et al., 1997). Roberts et al (2009) found that sediment pollution from fish farms can have adverse effects on benthic macroinvertebrates. An increase in suspended solids from fish farm effluents may cause a decrease in lake transparency (Guilpart et al., 2012). Previous studies (Wu et al., 2012; Silva et al., 2013; Karimi et al., 2016) have found that pollution of sediments by effluents from fish farms could have resulted in the reduction of macrobenthic community diversity.
However, the diversity (Shannon-Wiener diversity index) of the macrobenthic communities in Ge Lake had been increasing over the period during 2008 and 2012. Although the differences in macrobenthic community diversity between years were not statistically significant, benthic invertebrate diversity appears to be improving in response to efforts by the local government to reduce environmental pollutants in Ge Lake. In 2007, 2008, and 2009, the City of Changzhou implemented various phases of environmental mitigation following the drinking water crisis of 2007. These mitigations included reducing the scale of fish net-pen culture and ecological restoration projects. Based on the yearbook of the City of Changzhou in 2009, the area of fish net-pen cultures was reduced from 44.7 square kilometers, which represented 22.7 percent of the total area in Ge Lake in 2007, to 7.3 square kilometers in 2009. Water purification was found to be influenced by aquatic plant growth in the eastern section of Ge Lake during 2009 and 2010 (Wu et al., 2013). Furthermore, the water quality in Ge Lake was shown to be improved through the reconstruction of submerged macrophyte communities in the lake (Huang, 2011). The results of several studies (Scheffer, 1998; Jukka and Leena, 2003; Griffin et al., 2009) have indicated that macrophytes as an important component of lakes, absorb phosphorus in eutrophic lakes, restrain sediment to increase lake transparency, and supply complex habitats for macrobenthic communities. The periphyton layer covering macrophytes is probably a particularly important food source for benthic macroinvertebrates (Brönmark, 1989). Researchers (Lloret and Marin, 2009) have found that the benthic macroinvertebrate community and benthic macrophytes play a crucial role in absorbing surplus nutritive materials from water and keeping them in sediments.
Factors That Affected the Structure and Diversity of Macrobenthic Communities
The physical and chemical characteristics of water are important factors that can affect freshwater macrobenthic community structure and diversity (Nicola et al., 2010). In this study, WT, DO, conductivity, NH4 +-N, transparency and TP were observed to be the primary environmental variables that affected macrobenthic community structure and diversity in Ge Lake. The abundance of food (Humpesch, 1979), the temperature can also affect the structures of communities of macrobenthos (Courtney and Clements, 1998; Joydas et al., 2016). Conductivity has been reported as another principal variable that influences the numbers and distribution of oligochaetes (Achurra et al., 2015). The study (Li et al., 2017) found a significant decline in diversity of the benthic invertebrate community of Tai Lake since 1980’s, which could be associated to continuous exposure to ammonia over decades given different sensitivity of taxa to ammonia in Tai Lake, China. Transparency was found to affect Pielou’s evenness index of the macrobenthic communities in the study area. Lower transparency likely results in lower primary productivity, resulting in less secondary growth of benthic macroinvertebrates. Increasing concentrations of TP and TN promoted phytoplankton growth, leading to phytoplankton blooms, resulting in lower transparency and lower concentrations of DO near the lake bottom (Zeng et al., 2013). In addition, the trophic state index, which is a synthetic parameter, was significantly negatively correlated with Pielou’s evenness index (Table 3). The results obtained in Ge Lake confirmed results of previous studies showing that lower taxon diversity is often found in supereutrophic lakes, while greater diversity is found in mesotrophic lakes (Frouin, 2000). Trophic status is correlated with water chemistry and is an important factor influencing freshwater benthic macroinvertebrate communities (Nicola et al., 2010). Ge Lake was classified as a supereutrophic lake, and macrobenthic community diversity in the lake was determined to be low. Severe eutrophication exerts detrimental effects on macrobenthic assemblages due to greater nutrient concentrations, leading to increased phytoplankton growth and favoring cyanobacteria growth, resulting in hypoxia (Kilgour et al., 2008). The changes of the macrobenthic community assemblages in Ge Lake observed over the past several decades are consistent with increasing eutrophication of lakes. The dominant macrobenthic community taxa in Ge Lake shifted from bivalves in 1990 to the oligochaete L. hoffmeisteri at present. This shift is consistent with results from Dianchi and Tai Lakes in China (Wang et al., 2007; Cai et al., 2012). Furthermore, the water upstream of Ge Lake is eutrophic (Table 2), and the Beigan River is a tributary that flows into the lake. Therefore, it is necessary to control pollutants upstream of Ge Lake.
Previous studies have shown that MC-LR is a toxic derivative of cyanobacterial blooms (Qin et al., 2010; Freitas et al., 2014). Acute and subacute toxicity can be induced by MCs in freshwater ecological food webs (Ibelings and Chorus, 2007; Lahrouni et al., 2012). The present study demonstrated that MC-LR concentrations were higher at the four Ge Lake sites than at the Changdang Lake site (Table 2). MCs have an adverse effect on Mollusca and zooplankton (Chen et al., 2005; Krzton et al., 2017). Although the concentrations of MC-LR in Ge Lake did not exceed WHO standards (1.0 μg/L), their potential impact on aquatic animals and humans in the future cannot be ignored.
Due to the increasing impacts of anthropogenic activities on Ge Lake, its macrobenthic communities are threatened by environmental pollutants. The change in the macrobenthic community structure in Ge Lake since the 1990s has been enormous. Thus, strict control on water quality and the introduction of nutrients from rivers into Ge Lake is urgent and crucial for protecting water functions and biological diversity in the lake. Additionally, it is equally important to control cyanobacteria blooms in Ge Lake. Analyzing the factors affecting water quality and macrobenthic metrics and seeking methods for improving the water environment will be helpful for water quality management and ecological function recovery in Ge Lake.
Conclusions
The present study records new data on the structure and diversity of the macrobenthic community in Ge Lake from 2008 to 2012. Changes in the structure of macrobenthic communities in Ge Lake were huge. This present study found that the macrobenthic communities in Ge Lake exhibited much lower diversity than historical reports that could reflect the environmental conditions of the lake. WT, conductivity, NH4+-N and TP were the main environmental variables that influenced the structure and diversity of macrobenthic communities in Ge Lake. The effects of fish net-pen cultures on macrobenthic community structure and diversity need to be furtherly studied. The present study should provide useful basic data for water environmental protection and ecological restoration in the shallow lowland lakes.
Supporting Information
Table S1 Macrobenthic Animals Collected in Ge Lake (Ch: Gehu). Occurrence (%) Represents the Percentage of Sites at Which the Species were Collected During Sampling.
| Species | Occurrence (%) | Species | Occurrence (%) |
|---|---|---|---|
| Mollusca | Procladius sp. | 2.5 | |
| Alocinma longicornis | 5.0 | Cryptotendipes sp. | 5.0 |
| Parafossarulus striatulus | 2.5 | Tanytarsus sp. | 10.0 |
| Bellamya aeruginosa | 25.0 | Microchironomus sp. | 5.0 |
| Bellamya purificata | 45.0 | Glyptotendipes tokunagai | 2.5 |
| Corbicula fluminea | 5.0 | Dicrotendipes nervosus | 2.5 |
| Anodonta angula | 12.5 | Tanypus punctipennis | 40.0 |
| Hyriopsis cumingii | 2.5 | Microchironomus tener | 2.5 |
| Oligochaeta | Tanypus vilipennis | 2.5 | |
| Aulodrilus sp. | 2.5 | Chironomus salinarius | 2.5 |
| Tubifex sp. | 5.0 | Chironomus riparius | 5.0 |
| Limnodrilus grandisetosus | 2.5 | Chironomus dorsalis | 2.5 |
| Limnodrilus claparedianus | 15.0 | Chironomus lugubris | 2.5 |
| Limnodrilus hoffmeisteri | 97.5 | Others | |
| Limnodrilus udekemianus | 2.5 | Sinictinogomphus sp. | 2.5 |
| Branchiura sowerbyi | 40.0 | Caridina sp. | 12.5 |
| Chironomidae | Gammarus sp. | 5.0 | |
| Chironomus attenuatus | 15.0 | / | / |
Table S2 Pairwise Pearson correlations between environmental variables.
| WT | pH | DO | Conductivity | NH4 +-N | Chl a | Transparency | CODMn | TP | TN | STSI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 1.000 | ||||||||||
| pH | 0.332** | 1.000 | |||||||||
| DO | 0.222 | 0.530*** | 1.000 | ||||||||
| Conductivity | 0.461*** | 0.524*** | 0.214 | 1.000 | |||||||
| NH4 +-N | -0.262* | -0.340** | -0.467*** | -0.241 | 1.000 | ||||||
| Chl a | 0.198 | -0.122 | -0.266* | -0.012 | 0.050 | 1.000 | |||||
| Transparency | 0.120 | 0.271* | 0.378** | -0.074 | -0.458*** | -0.147 | 1.000 | ||||
| CODMn | -0.316* | -0.285* | -0.535*** | 0.039 | 0.274* | 0.313* | -0.550*** | 1.000 | |||
| TP | -0.041 | -0.129 | -0.291* | 0.180 | 0.323* | 0.021 | -0.562*** | 0.504 | 1.000 | ||
| TN | -0.101 | -0.444*** | -0.302* | 0.046 | 0.084 | 0.307* | -0.510*** | 0.474 | 0.469 | 1.000 | |
| STSI | -0.137 | -0.321* | -0.510*** | 0.064 | 0.365** | 0.523*** | -0.761*** | 0.790*** | 0.692*** | 0.736*** | 1.000 |
| MC-LR | -0.074 | 0.168 | 0.157 | 0.042 | -0.023 | 0.412* | 0.186 | 0.468* | 0.375* | 0.057 | 0.378* |
*: P<0.05; **: P<0.01; ***: P <0.001. Note: STSI was excluded due to its higher correlations with Transparency and CODMn (bold numbers).

Figure S1 Mean Values (n=10) of the Shannon-wiener Diversity Index based on the Macrobenthic Communities at Six Sampling Sites (S1, S2, S3, S4, S5 and S6) in Ge Lake in Eastern China.











 nueva página del texto (beta)
nueva página del texto (beta)