Introduction
Traditional nitrogen removal biotechnologies take a long process. But some researchers show that autotrophic denitrification can be performed by a Sequence Batch Biofilm Reactor (SBBR). Because this is done in a single reactor, rapidly, and using simple techniques, it has become a subject of great interest in the field of biological nitrogen removal (Ahn, 2006; Jetten et al., 2005). The prevailing view holds that the main functional bacteria in this process are ammonia oxidizing bacteria (AOB), nitrite oxidizing bacteria (NOB) and anaerobic ammonium oxidizing bacteria (ANAMMOX) (Dong & Tollner, 2003; Sliekers et al., 2002; Third, Sliekers, Kuenen, & Jetten, 2001). Ammonium is partially oxidized by AOB to nitrite, which is subsequently consumed by ANAMMOX together with the remaining ammonium. However, AOB and NOB are aerobic bacteria while ANAMMOX are obligate anaerobes, which do not tolerate dissolved oxygen (DO) (Strousm, Vanger, Kuenen, & Jetten, 1997). Therefore, the key to one-step autotrophic denitrification in a single reactor is to balance the supply and demand of dissolved oxygen (Qin, Guo, Fang, & Yang, 2009; Hippen, Rosenwinkel, Baumgarten, & Seyfried, 1997; Galluzzo, Ducato, Bartolozzi, & Picciotto, 2001) and how to make them coexist in one reactor.
In this study, autotrophic nitrogen removal was conducted in an SBBR (Guo, Qin, Fang, & Yang, 2008). Previous research has shown that autotrophic nitrogen removal process has been set up and microbial community structure exerts significant influence on the performance of this system (Fang, Qin, Guo, Jia, & Yang, 2010). Microbes are at the core of the reactor, and the denitrification effect of activated sludge and bio-film is directly determined by the composition of the microbial community. This is a comparative study of activated sludge and biofilm in an SBBR, with quantitative analysis of functional bacterial populations as well as nitrogen transfer pathways. This study is to analyze the different roles between activated sludge and biofilm in autotrophic nitrogen process.
Materials and methods
Experimental apparatus
A schematic view of the SBBR autotrophic denitrification reactor is shown in figure 1. The reactor was filled with hollow balls fillers and soft combination packing. Under the condition of 30 °C and pH of 8.0, artificial synthetic ammonia nitrogen wastewater was added in. The concentration of NH4HCO3 was 160 mg/l without organic carbon sources. According to the optimal operation condition about 2h: 2h aeration/non-aeration ratio and DO 2.0 (aeration) mg/l /0.4 (non-aeration) mg/l, after half year domestication, autotrophic nitrogen removal system was set up successfully. TN removal rate reached to 80% while ammonia conversion rate reached to 100%.
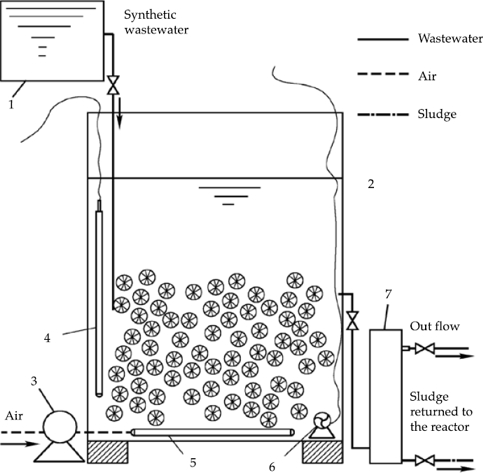
Figure 1 Schematic representation of the reactor and workflow. 1. Wastewater tank; 2. SBBR reactor; 3. Air pump; 4. Calorstat; 5. Aerating stick; 6. Stirrer; 7. Effluent tank.
Nitrosation and anaerobic ammonium oxidation were tested separately in both activated sludge and biofilm samples in the SBBR. Other tests were done in conical flasks (figure 2), which were sealed with rubber plugs to maintain a stable DO concentration. Artificial wastewaters with different NH4+-N or NO2--N concentrations were added.
Experimental apparatus
(1) Quantitative analysis of functional bacteria
DNA was extracted using a Shanghai Biocolors BioScience and Technology Company K717 environment genomic DNA extraction kit. Quantitative real-time PCR was carried out using SYBR Green Real-time PCR Master Mix Kit (Bio-Rad) in an iCycler IQ® Multiplex Real-Time PCR DNA System (Bio-Rad). A melting curve analysis was performed for each sample after PCR amplification to ensure that a single product with the expected melting curve characteristics had been obtained. The recombinant plasmid vectors were constructed by standard procedures.
(2) Nitrosation rate
Activated sludge and biofilm samples were taken from an SBBR which was performing effective and stable autotrophic nitrogen removal. The samples were diluted with distilled water and then inoculated into two 250 ml flasks, into which had already been added 100 ml of artificial wastewater. The initial concentration of NH4HCO3 was 70 mg/l, and pH was adjusted to 8.0 (± 0.2) by the addition of NaHCO3. The nutrient solution with trace elements such as Fe, Mg was also added (EDTA 5.0 g/l, CoCl2·6H2O 1.6 g/l, ZnSO4·7H2O 2.2 g/l, MnCl2·4H2O 5.1 g/l, CuSO4·5H2O 1.6 g/l (NH4)6Mo7O24·4H2O 1.1 g/l, CaCl2·2H2O 5.5 g/l, FeSO4·7H2O 5.0 g/l). The flasks were maintained at a temperature of 30 °C using a heated magnetic stirrer. DO concentration was controlled to 2.0 mg/l by aerating with a mixture of O2 and Ar. The flasks were placed in a constant temperature shaking reactor at 30 °C and shaken at 150 revs/min.
Concentrations of NH4+, NO2-, NO3- and TN were measured at intervals during each 48 h test to obtain concentration curves. The maximum reaction rate Vmax was calculated from the maximum slope of each concentration curve. At the end of each test, activated sludge or biofilm samples were taken out of the flask by brushing with a sterile brush and rinsing with distilled water, and volatile suspended solids (VSS) were measured:

(3) Anaerobic ammonium oxidation rate
The experimental procedure and analysis was similar to the study of nitrosation rate, except for the components of artificial wastewater and DO concentration. NH4HCO3 and NaNO2 concentrations in the artificial liquid were both 70 mg/l. The liquid was sparged with Ar gas for 30 min to remove DO to below the detection limit (0.01 mg/l) of an LDOTM HQ10 dissolved oxygen meter.
Results and discussion
Identification of recombinant plasmid
The recombinant plasmids sequences were analyzed using a GenBank BLAST search. As shown in table 2, conservative regions of 16S rDNA of functional bacteria were obtained. These recombinant plasmids were suitable for quantitative analysis of AOB, NOB, and ANAMMOX in real-time PCR experiments.
Quantitative analysis of AOB, NOB, and ANAMMOX
Quantitative real-time PCR results are shown in table 3. The populations of the three main functional bacteria (ammonia oxidizing bacteria AOB, nitrite oxidizing bacteria NOB and anaerobic ammonium oxidizing bacteria ANAMMOX) in activated sludge and biofilm samples were significantly different. That is to say that in this autotrophic nitrogen removal system, activated sludge and biofilm contained different communities of functional bacteria. This suggests that activated sludge and biofilm play different roles in the overall nitrogen removal procedure.
AOB
In the first step in the nitrogen removal process, ammonium was oxidized to nitrite by AOB. The population of AOB in the activated sludge sample was 1.88 × 1011 cells/g, almost nine times higher than the 1.90 × 1010 cells/g found in the biofilm. AOB are typically aerobic bacteria which thrive under high DO. Activated sludge was suspended in the reactor and was in intimate contact with DO. However, poor mass transfer of DO, into the biofilm meant that the interior layer of biofilm was anaerobic or at least had low DO concentration. Therefore AOB tended to be found in activated sludge or the exterior layer of biofilm.
NOB
NOB numbers were 2 to 4 orders of magnitude less than AOB and ANAMMOX in both activated sludge and biofilm samples. NOBs were not the dominant bacteria in terms of cell number in this SBBR reactor although they multiplied faster than AOB under normal conditions (5-20 °C) (Hellinga, Schellen, Mulder, & Heijnen, 1998). Other studies have also shown that at higher temperatures (> 20 °C), the degradation rate of AOB can be higher than that of NOB (Hellinga et al., 1998). During the start-up period of this study, the temperature was kept around 30 °C so that NOB was depleted and AOB became be the dominant group to accomplish one-step autotrophic nitrogen removal and prevent nitrite from being oxidized to nitrate.
ANAMMOX
The population of ANAMMOX was 5.5 times that of AOB and 874 times that of NOB in activated sludge samples. These differences were more pronounced in biofilm samples, at 140 times and 3491 times, respectively. Jetten et al. (2001) reported that anaerobic ammonia oxidation was achieved only when the ANAMMOX population was higher than 1010-1011 cells/ml. Therefore, although ANAMMOX cells were the most abundant one among three functional bacteria in this study, they did not dominate the metabolic functionality. AOB and NOB are an aerobic bacteria, but ANAMMOX are anaerobic and are sensitive to DO. They can not survive even at 0.5-2.0% air saturation. The low rate of mass transfer of DO in biofilm and depletion by AOB and NOB in the outer layer made the interior of the biofilm an ideal condition for ANAMMOX. As a result, the population of ANAMMOX in biofilm was 2.66 × 1012 cells/g, which was 2.6 times that in activated sludge.
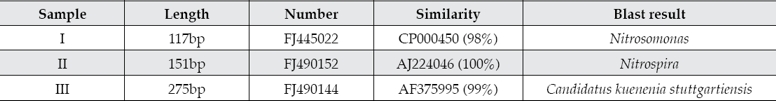
Nitrosation of activated sludge and biofilm
Transformation of nitrogen in activated sludge and biofilm samples in a cycle of nitrosation are shown in figures 3a and 3b.
Figure 3a shows that ammonia can be fully oxidized by activated sludge in a single 48 h cycle, with the ammonia oxidation rate reaching 100%. The maximum rate occurred between the 6 and 20 h with a maximum ammonia oxidation rate of 0.23 mgN mgVSS−1 d−1. 85% of the oxidized ammonia was present as NO2--N and 9% as NO3--N. There was the limited removal of TN. The activated sludge in this system showed good ammonia oxidation activity under aerobic conditions. Nitrogen transformation by biofilm during a 48 h cycle is shown in figure 3b. The ammonia oxidation rate was 72%. Compared with nitrosation by activated sludge, there was no obvious inflection point on the ammonia concentration curve with biofilm. The maximum rate of ammonia oxidation rate by biofilm was 0.08 mgN mgVSS−1 d−1. The nitrosation activity of activated sludge was about 2.89 times that of the biofilm. Ammonia was mainly oxidized to NO2--N and the maximum concentration of NO3--N was only 4 mg/l at the end of the reaction cycle. The TN removal rate was 6.7%.
Both activated sludge and biofilm showed high nitrosation activities and there was little NO3--N accumulation in either system because they were acclimated to nitrosating conditions at the start of the experiment. NOB had been depleted and so most of the oxidized ammonia existed as nitrite. There were 158 times as many AOB as NOB cells in activated sludge samples while the ratio was 25 times in the biofilm sample. Accordingly, the aerobic ammonia oxidation rate of biofilm was less than that of activated sludge. This also led to a difference in nitrosation capacity between the two samples. This experiment used real-time PCR technology for bacterial quantification. Results showed that the populations of AOB were 1.88 × 1011 cells/g in the activated sludge samples and 1.90 × 1010 cells/g in biofilm samples. Cell counts in the sludge sample were about ten times those in the biofilm. AOB, which were responsible for aerobic ammonia oxidization, were mainly found in activated sludge in this SBBR autotrophic nitrogen removal reactor.
Studies have shown that there might be denitrification under aerobic conditions by aerobic denitrifiers (Robertson & Kuenen, 1990; Chen, Xia, & Ju, 2003; Hung, Mitsuyo, & Makoto, 2005). However, in this study, aerobic TN removal rates of activated sludge or bio-film samples were both low. After 48 h, the TN removal rates of sludge and biofilm samples were only 6.7 and 5.3% respectively. The NH4+ to NO2- conversion rates of sludge and biofilm samples were 85 and 83%, respectively. The concentration of NO3- was low, with the highest concentrations seen at the end of the 48 h experiment cycle of just 6.7 and 4.0 mg/l. Little aerobic denitrification was seen in the SBBR autotrophic nitrogen removal process. ANAMMOX activity was limited by continuous aeration to maintain a DO concentration of 2.0 mg/l, but ANAMMOX which survived in the activated sludge flocs and the interior of biofilm might provide the system with some anaerobic nitrogen removal capability.
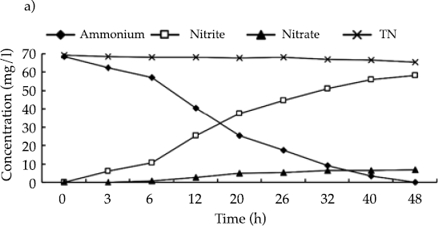
Anaerobic ammonium oxidation of activated sludge and biofilm
Figures 4a and 4b show the anaerobic ammonium oxidation activities of activated sludge and biofilm samples. Initial concentrations of NH4+ and NO2- in the artificial wastewater were both 70 mg/l. TN concentrations in both samples declined over the 48h experimental period. TN removal rates were 37% by activated sludge and 83% by biofilm. In contrast with nitrosation activity, it was confirmed that TN was removed mainly by anaerobic ammonium oxidation in this reactor. The reaction rate of anaerobic ammonium oxidation by activated sludge was 0.09 mgN mgVSS−1 d−1. This was just 41% of the rate seen in biofilm, which was 0.22 mgN mgVSS−1 d−1.
The concentration of NO3- in the activated sludge treatment varied between 15.0 and 17.4 mg/l, and in the biofilm treatment, the range was 13.8 to 18.0 mg/l, though no NO3--N was added to the artificial wastewater. The initial concentrations of NO3- in activated sludge and biofilm treatments were 15.0 and 13.8 mg/l, respectively. One possibility is that NaNO2- was unstable and was partially oxidized to NaNO3, which provided the initial NO3-. Figures 4a and 4b show that the concentrations of NO3- did not vary greatly over the course of the experiments. The small fluctuations that were observed may have been caused by measurement error. Van de Graaf et al. (1995) concluded that ANAMMOX could utilize both NO3- and NO2- in the anaerobic ammonium oxidation reaction because both can function as electronic receptors to convert the remaining NH4+ into N2. When the substrate contained both NO3- and NO2-, it was the NO2- that acted as the main electronic receptor for ANAMMOX. In this study, the removal efficiency of TN was high and the largest consumption rates of NH4+ and NO2-in activated sludge were 0.05 and 0.06 mgN mgVSS−1 d-1, respectively while for biofilm the values were 0.12 and 0.14 mgN mgVSS−1 d−1. The concentration of NO3-in the system was more-or-less constant, which suggests that NO2- was the main electronic receptor in anaerobic ammonium oxidation in this SBBR system. This was in accordance with Van de Graaf’s findings.
Conclusions
Activated sludge and biofilm played different roles in autotrophic nitrogen removal process.
Both activated sludge and biofilm samples exhibited nitrosation activity in the SBBR autotrophic denitrification system, the maximum ammonia oxidation rates of activated sludge and biofilm were 0.23 and 0.08 mgN mgVSS−1 d−1, respectively. The nitrosation activity of activated sludge was about 2.89 times that of the biofilm. The populations of AOB in activated sludge and biofilm were 1.88 × 1011 and 1.90 × 1010 cells/g, respectively.
The maximum anaerobic ammonia oxidation rate of activated sludge was 0.09 mgN mgVSS−1 d−1 , just 41% of the maximum rate of biofilm, which was 0.22 mgN mgVSS−1 d−1 . Anaerobic ammonia oxidation occurred mainly in the biofilm, with ANAMMOX being the dominant bacteria in this process. The populations of ANAMMOX were 1.04 × 1012 cells/g in activated sludge and 2.66 × 1012 cells/g.
TN removal rates of sludge and biofilm samples were just 6.7 and 5.3%, respectively, under aerobic conditions with both NO2- and NO3- present. No significant aerobic denitrification was seen in this SBBR autotrophic nitrogen removal system. TN removal in this study was performed mainly by ANAMMOX under anaerobic condition.











 nueva página del texto (beta)
nueva página del texto (beta)