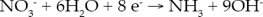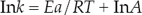Introduction
Groundwater has become increasingly polluted with nitrate nitrogen as a result of rapid industrial and agricultural development. Nitrate pollution increases the concentration of nitrogen in water, resulting in eutrophication. In the human body, nitrate is converted to nitrite, which is highly toxic (Fan, Qu, Liu, & Meng, 2000; Kapoor & Viraraghavan, 1997; Chew & Zhang, 1998; Michael & Michellem, 2002). Therefore, the removal of nitrate from water is of great practical significance. The methods currently used to remove NO3- from surface water primarily include ion exchange, reverse osmosis, biological treatment, and chemical reduction. The repeated regeneration processes that occur during ion exchange and reverse osmosis produce secondary pollution. Likewise, biological treatment entails numerous operation and management requirements, including a sufficient carbon source. Compared to ion exchange, reverse osmosis, and biological treatment, the chemical reduction has many advantages, such as rapid reaction rates. In addition, the chemical reduction does not cause secondary pollution, is not difficult to perform and is suitable for the treatment of small and distributed areas (Chen & Wu, 2009; Fan, Guan, Ma, & Ai, 2009). Kinetics and corrosion products of aqueous nitrate reduction by iron powder without reaction conditions control (Journal of Environmental Sciences, 2009, 21, 1028-1035).
Since the 1990s, numerous studies concerning the removal of nitrate-nitrogen pollution from surface water using nanoscale iron as an adsorbent and reductant have been conducted (Song & Song, 2015; Chen & Wu, 2009; Fan et al., 2009; Cheng, Muftikian, Fernando, & Korte, 1997; Huang, Wang, & Chiu, 1998; Choe, Chang, Hwang, & Khim, 2000; Lien & Zhang, 2001; Ponder, Darab, & Mallouk, 2000). Nanoscale iron has a large surface area (Liou, Lo, & Lin, 2005; Philips & Laura, 1992; Siantar, Schreier, Chou, & Reinhard, 1996; Wang & Zhang, 1997; Kanel, Manning, Charlet, & Choi, 2005; Yuan & Lien, 2006; Lien, Jhuo, & Chen, 2007; Tratnyek, Johnson, & Scherer, 1996), good surface adsorption, and strong reduction capabilities (Murphy, 1991). Previous studies have shown that 4 g/L of nanoscale iron can efficiently and effectively remove 30-120 mg/l of nitrate nitrogen. In one study, Seunghee Choe (Seunghee, Howard, & Li, 2004) investigated the effects of pH on the reaction rate of nitrate nitrogen in a nanoscale iron removal solution. The results indicated that nitrate nitrogen can be removed entirely under acidic conditions. In another study, Liou (Liou et al., 2005) studied the effects of temperature on the reaction rate of nitrate nitrogen as well as the nitrate removal efficiency of nanoscale iron. Furthermore, Chunming (Chunm & Robert, 2004) investigated the influential factors and reaction products of nanoscale iron-facilitated nitrate-nitrogen removal in different acidic solutions. In all of these studies, the influential factors and nitrate-nitrogen removal efficiency of nanoscale iron were investigated using relatively large amounts of nanoscale iron. In this study, the influential factors and nitrate-nitrogen removal efficiency of small amounts of nanoscale iron are analyzed in order to facilitate the efficiency of nitrate removal in practical applications. In addition, the reaction kinetics of the removal process were studied in order to develop a kinetic equation that effectively describes the reaction rate and activation energy of nanoscale iron-facilitated nitrate-nitrogen removal and, thereby, provides a theoretical basis for the application of nanoscale iron technology to the treatment of wastewater.
Materials and methods
Instruments and reagents
The reagents used for the purposes of this study included KNO3 (analytical reagent grade, A.R.), FeSO4·7H2O (A.R.), NaOH (A.R.), KBH4 (A.R.), absolute ethyl alcohol (A.R.), polyethylene glycol (A.R.), and reduced iron powder (200 mesh).
In addition, the D/MAX-2500 X-ray diffractometer (Japan), Philips EM400ST transmission electron microscope, 752N UV-Vis spectrophotometer, DJIC-100 electric mixer, Formal1025 anaerobic operation box, THZ-82B air bath constant-temperature-oscillator, JW-04 nitrogen adsorption surface area tester, and Delta-320 pH meter were used to conduct the experiments (Song & Song 2015).
All of the glassware was immersed in 10% (volume fraction) HNO3 for 48 hours and then rinsed with tap water and deionized water several times before use.
Preparation and characterization of the nanoscale iron
At room temperature, a 1.6 mol/l NaBH4 solution was added drop by drop to the same volume of 1.0 mol/l FeCl3 solution; during this process, a magnetic stirrer was used to stir the mixture. Then after ten minutes of reaction, we obtained the nanoscale iron particles needed for the experiment. According to reaction equation (1), this process resulted in the reduction of Fe3+ to nanoscale iron (Zhang, Wang, & Lien, 1998):
The black iron grains obtained via the above method have washed a minimum of three times with de-ionized water and absolute alcohol and then dried for 4 hours at 100-105 ˚C. The samples were then preserved in the dry container. All of these processes were conducted under nitrogen protection conditions.
The specific surface area and porosity analyzer were used to measure the specific surface area of the nanoscale iron particles via the nitrogen adsorption method. The X-ray diffractometer was used to perform a phase analysis of the nanometer particles with Cu as the target, Ka as the ray, and 100 mA as the current flow rate. The scanning transmission electron microscope was used to observe the features of the particles, and the nitrogen adsorption surface area tester was used to determine the specific surface areas of the particles.
Water sample analysis method
The methods adopted in the water sample analysis experiments included the nitrate nitrogen test method (UV spectrophotometry), the subnitrate nitrogen test method (N-(1-naphthyl)-ethylenediamine photometry), and the ammonia nitrogen test method (Nessler reagent spectrophotometry).
Nitrate nitrogen removal method
First, 5 ml of nitrate nitrogen solution with a known concentration and nanoscale iron were added to a 250-ml conical flask and allowed to react in the water-bathing constant temperature vibrator at a rotation speed of 150 rpm. The water-bathing constant temperature vibrator was used to control the reaction temperature. Next, after time sampling, each sample was filtered using a 0.45-μm membrane. Then, the concentration of each sample was measured.
Results and discussion
Characterization analysis results
According to the results, the diameter of the nano-iron particles ranged from 20 nm to 60 nm. In addition, the synthesized particles primarily existed in the granular and linear states with numerous gaps, indicating that the absolute alcohol controlled the accumulation of the nanoscale iron parcels during the synthesis process. Therefore, the absolute alcohol significantly increased the specific surface area of the nanoscale iron particles (Wang, Jin, Li, Zhang, & Gao, 2006). The average specific surface area of the synthesized nanoscale iron parcels was approximately 41.16 m2/g, 1 to 2 orders of magnitude higher than that of the micro iron particles available for purchase. The results obtained via the X-ray diffractometer (figure 1) indicated that when the scanned diffraction angle (2θ) ranged from 30° to 100°, the synthesized nanoscale iron exhibited diffraction peaks at 44.58, 64.03, 81, and 89°, corresponding to the diffraction peaks of the body-centered cubic a-Fe (110). Furthermore, diffraction peaks approximate to those of the body-centered cubic a-Fe (200) and a-Fe (211) were observed (figure 1), indicating that the particles prepared through these experiments were comprised of iron rather than iron oxide.
Influence of the initial nitrate nitrogen concentration on the removal efficiency
At a constant temperature of 25 °C, 0.5 g of nanoscale iron was added to water containing 10, 50, and 100 mg/l of nitrate nitrogen. Then, the nitrate nitrogen concentrations at different reactions were measured with the spectrophotometer. The reaction time was used as the abscissa and the nitrate removal efficiency was used as the ordinate. The effects of the initial nitrate nitrogen concentration on the removal efficiency were investigated, as shown in figure 2. As shown in this figure, as the initial concentration decreased, the removal rate increased and the ratio of the final concentration to the initial concentration decreased, with ratios of 0.69, 0.67, and 0.64. The removal rate was highest when the nitrate nitrogen concentration was 10 mg/l. In addition, the reaction rate became constant after 30 minutes regardless of the initial nitrate-nitrogen concentration. The experimental results indicated that the initial nitrate nitrogen concentration affected the reaction rate, but not the removal rate. In nanoscale iron-facilitated nitrate-nitrogen removal, the nanoscale iron first absorbs the nitrate nitrogen. Then, the nitrate nitrogen is converted into nitrite nitrogen, ammonia, and trace amounts of nitrogen via chemical reactions on the surface of the nanoscale iron (Zhang, Jin, Han, & Qin, 2006). The adsorption and response capacities of nanoscale iron are constants. As a result, the nanoscale iron content was relatively excessive when the nitrate nitrogen concentration was low, resulting in complete adsorption and conversion. Likewise, the nanoscale iron content was relatively inadequate when the nitrate nitrogen content was high, resulting in incomplete absorption and conversion.
Influence of pH on the removal efficiency
Iron forms ions easily in acidic solutions and combines with hydroxide ions to form precipitates in alkaline solutions. Therefore, the effects of pH on the nitrate-nitrogen removal efficiency were also investigated. In the experiment, 0.5 g of nanoscale iron was added to water containing 50 mg/l of nitrate nitrogen at a constant temperature of 25 °C. Next, dilute HCl and NaOH were used to adjust the pH of the solution to values of 2.0, 4.0, 6.0, 7.0, 8.0, and 10.0. The nitrate nitrogen concentration was then measured after different reaction times.
The results are shown in figure 3. As shown in this figure, the ratio of the final nitrate nitrogen concentration (C) to the initial nitrate nitrogen concentration (C0) of the solution increased gradually as the pH increased. In addition, the maximum removal efficiency occurred when the pH of the solution was 2.0. As the pH increased, the removal rate also gradually increased. After achieving equilibrium, the highest nitrate-nitrogen removal rate was only approximately 20%. This was attributed to the conversion of zero-valent iron to iron ions. The reaction equation can be expressed as:
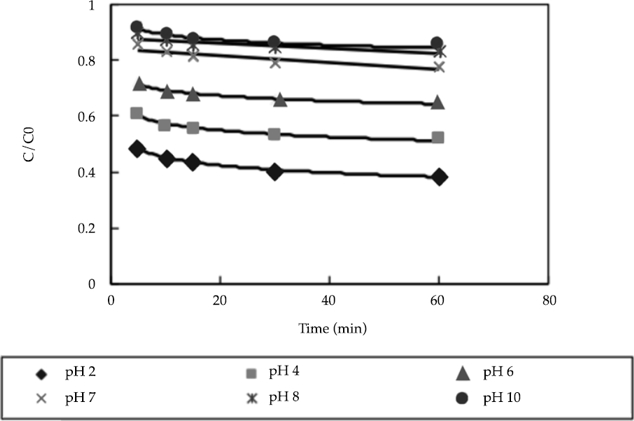
Figure 3 Influence of different pH values on removal efficiency (in the experiment, 0.5 g of nanoscale iron was added to water containing 50 mg/l of nitrate nitrogen at a constant temperature of 25 °C).
As shown in the following equation, the zero-valent iron also reacted with the nitrate nitrogen:
Furthermore, under acidic conditions, the zero-valent iron reduced the nitrate nitrogen according to the following reaction equation:
In an alkaline environment, Fe0 is easily converted into Fe(OH)2, Fe(OH)3, and several ferrous hydroxide complexions, such as [Fe(OH)]+, [Fe(OH)3]-, [Fe(OH)4]2-, and [Fe(OH)4]2+. These changes significantly reduce the ability of iron to absorb nitrate nitrogen (Martin et al., 2008; Yang & Lee, 2005). Due to the dual role of adsorption and reduction during nanoscale iron-facilitated nitrate-nitrogen removal, the pH of the solution significantly impacted the reactions. Since the acidity of water in practical engineering applications varies, adjusting the pH of water could allow for improved nitrate-nitrogen removal.
Influence of temperature and kinetic analysis of the removal of nitrate-nitrogen
In the experiment, 0.5 g of nanoscale iron was added to water containing 50 mg/l of nitrate-nitrogen. Then, the nitrate nitrogen content was measured at 25, 30, 35, 40, 45, and 50 ˚C. The results are shown in figure 4. As shown in this figure, the nitrate nitrogen content varied at different temperatures, with nitrate removal rates of 50 and 60% at 25 °C and 50 °C, respectively. Thus, as the temperature increased, the amount of nitrate nitrogen removed by the nanoscale iron increased. However, these effects were not significant. Thus, efficient nitrate-nitrogen removal can be achieved at various temperatures in practical engineering applications. Figure 4 also shows that the reaction rate decreased gradually over time, but became constant after approximately 10 minutes. Therefore, the reaction that occurred between the nanoscale iron and nitrate nitrogen cannot be determined with this information alone. However, the products of the reaction between the nanoscale iron and water as well as the conditions of the aqueous solution determined the products of nitrate reduction. Thus, the reaction that occurred between the nanoscale iron and nitrate nitrogen under different conditions can be described by the following equation:
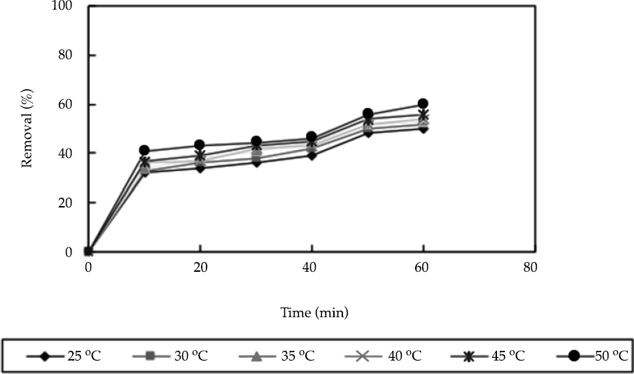
Figure 4 Influence of different temperature on removal efficiency (in the experiment, 0.5 g of nanoscale iron was added to water containing 50 mg/l of nitrate-nitrogen).
Since the removal of nitrate-nitrogen occurs via adsorption and reduction facilitated by both the large specific surface area and high activity of nanoscale iron, the reaction between nitrate nitrogen and nanoscale iron cannot be effectively described by an adsorption reaction equation alone. Previous studies concerning the kinetics of nanoscale iron-facilitated nitrate-nitrogen removal have yielded varying results. Liou (Liou et al., 2005) reported that the reaction between nanoscale iron and nitrate nitrogen can be described with a pseudo-first-order kinetic equation. In contrast, Gordon (Molly et al., 2003) claimed that this reaction cannot be described by a pseudo-first-order kinetic equation or first-order kinetic equation. Since the effects of adsorption and reduction and interrelationships on nanoscale iron-facilitated nitrate-nitrogen removal cannot be determined, the products and reactant concentrations are assumed to be influenced by the conditions under which the reaction occurs. Thus, there is no universally accepted kinetic equation for the reaction between nanoscale iron and nitrate nitrogen. In this paper, a pseudo-second-order kinetic equation was developed in order to determine the value of the reaction rate constant at different temperatures. Since the nitrate removal rate in this study ranged from 50 to 60%, not appropriate to research its kinetics equation from the half-life point of view. The data also indicated that the reaction became constant after 10 minutes due to the increased concentration of reacted nitrate nitrogen and resulting decrease in the reaction rate. The following pseudo-second-order kinetic equation was used to describe the reaction between the nitrate nitrogen and nanoscale iron:
In this equation, Ci represents the difference between the initial nitrate concentration C0 and the nitrate concentration at time t Ct (mg/l), Ca represents the difference between the initial nitrate concentration C0 and the stable nitrate concentration Ce (mg/l), and k is the reaction rate constant (mg/(l·min). This equation was integrated and simplified as:
Equation (7) was used to calculate the value of k. Then, the appropriate value of k was selected based on the statistical error between the fitted value and experimental data. The results are shown in figure 5 and table 1.
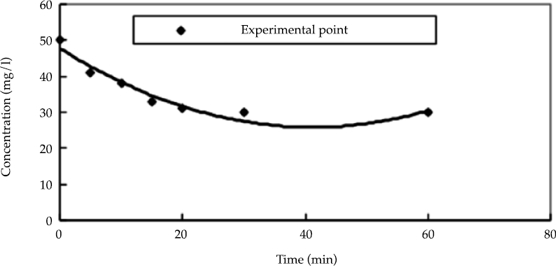
Figure 5 Kinetics analysis of experimental data according to pseudo-second-order at 25 ˚C (the following pseudo-second-order kinetic equation was used to describe the reaction between the nitrate nitrogen and nanoscale iron: Ct = C0 - ktC2a / 1 + ktCa).
Temperature also affects the reaction rate. According to the Arrhenius equation:
An lnk 1/T relationship diagram was constructed in order to determine the activation energy, as shown in figure 6. According to the data presented in figure 6, the activation energy Ea of the reaction was approximately 17.18 kJ/ mol. First, the nitrate nitrogen was absorbed by the nanoscale iron. Next, the nitrate nitrogen reacted with the surface of the nanoscale iron. Then, a fraction of the zero-valent nanoscale iron was converted into divalent iron ions. Therefore, the Ea included the total amount of reduced nitrate and oxidized nanoscale iron. Since the activation energy of the reaction, which was primarily influenced by the mass transfer of the aqueous solution, was limited to 10-20 kJ/mol, the factor that primarily influenced the reaction was the mass transfer of the solution rather than the chemical reactions (Liou et al., 2005). These results corresponded with those reported by Liou, who used a nanoscale iron to remove nitrate-nitrogen at 10-60 ºC (Liou et al., 2005).
Product analysis of the nitrate-nitrogen removal process
At a constant temperature and pH of 25 ºC and 7.0, respectively, 0.5 g of nanoscale iron was added to water containing 50 mg/l of nitrate nitrogen in order to determine the nitrate nitrogen, nitrite nitrogen, and ammonia concentrations of the solution after different reaction times. The results are shown in figure 7. As shown in figure 7, chemical reactions occurred during the nanoscale iron-facilitated removal of the nitrate-nitrogen. The nitrite content was very low. According to the curves of the three nitrogenous compounds, the reactant concentration decreased from the initial concentration of 50 to 45 mg/l, indicating that a portion of the nitrate nitrogen was converted into nitrogen and then released.
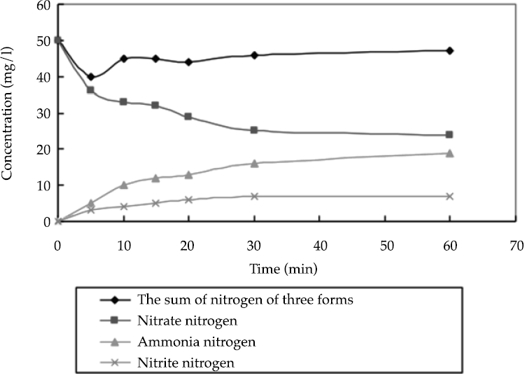
Figure 7 Production of nitrate´s deoxidization by nanoscale iron (at a constant temperature and pH of 25 ˚C and 7.0.).
According to the experimental results, during the reaction between the nitrate nitrogen and nanoscale iron, most of the nitrate nitrogen was converted to ammonia and nitrite nitrogen, which were later removed. As shown in equations (5) and (6), under the neutral reaction conditions, most of the nitrate nitrogen was converted to ammonia. Thus, the nitrate nitrogen was primarily reduced to ammonia. The standard electrode potential of the Fe0 and its oxidation-reduction reaction with Fe2+ within the liquid solution (Fe0/Fe2+) was approximate -0.440 V, indicating that Fe0 is a strong reducing agent of substances that can be easily reduced, such as H+, CO32-, SO42-, NO3-, and O2 (Schlieker et al., 2000). In the Fe-NO3- system, Fe0 acts as the reductant and loses electrons, while NO3- acts as the oxidant and gains electrons. The NO3- gains electrons released by the Fe0 as well as the oxidation products of the Fe, including Fe2 + and H2. According to thermodynamics, Fe0 could be converted into Fe2+ after losing electrons, and NO3- is reduced to N2. Therefore, Fe0 can completely reduce NO3-, but the reduction products of NO3- are determined by the reaction conditions (Agrawal & Tratnyek, 1996).
Conclusions
When nitrate-nitrogen removal was performed with different concentrations of nanoscale iron, lower nitrate nitrogen concentrations were associated with increased removal. This was primarily influenced by the adsorption site of the nanoscale iron.
Under acidic conditions, the nanoscale iron exhibited the highest adsorption ability and maximum nitrate-nitrogen removal when the pH of the solution was 2.0.
The temperature of the solution influenced the reaction rate. The kinetic equation Ct = C0 - ktC2a /1 + ktCa was developed based on the experimental data. According to the equation, the reaction rate constant k was highest with a value of 0.014 mg/(l/min) at 50 ˚C.
By calculating the value of the reaction rate constant k at different temperatures, the reaction activation energy Ea was determined to be approximately 17.18 kJ/mol. The results indicated that the reaction was primarily affected by the mass transfer of the solution.
Under neutral conditions, the reaction product of the nitrate nitrogen solution was ammonia.











 text new page (beta)
text new page (beta)