Introduction
Heavy metals are harmful to aquatic ecosystems, and remain in the environment for a long time (Ameh & Akpah, 2011; Bissenbaev, Ishchenko, Taipakova, & Saparbaev, 2011; Tanhan, Kruatrachue, Pokethitiyook, & Chaiyarat, 2007). Copper is a toxic heavy metal pollutant that quickly accumulates in animals and humans through the food chain, which seriously affects the metabolism of the human body. The human body releases Cu2+ very slowly, causing damage to bodily organs that are irreversible. Therefore, Cu2+ pollution treatment has become an urgent subject. Traditional methods of treatment to heavy metal pollution in soil mainly use the mixing of soil, leaching method, chemical modifiers, and so on. Not only are these physical and chemical methods expensive, they cannot be applied into small areas and cause second pollution. Such methods cannot fundamentally solve the Cu2+ pollution in soil. (He, Huang-Xiao, & Chen, 2011; Visioli & Marmiroli, 2013; Mirza, Hossain, & Masayuki, 2010). In recent years, people have found the bioconcentration ability of some plants to heavy metals. It is a high efficiency, environmental protection and cost control measures to use such plants as phytoremediation of heavy metal pollution. This treatment measure has very broad application prospects (Engelen, Sharpe-Pedler, & Moorhead, 2007; Chaumont et al., 2012; Rajkumar, Sandhya, Prasad, & Freitas, 2012; Zhou et al., 2007).
The calamus (Acorus calamus L.) and reed (Phragmites australis) are common across China’s natural wetlands. The high Cu2+ absorption features of these two plants species provide scientific basis for phytoremediation of heavy metal pollution in water bodies.
Materials and methods
Acorus calamus and Phragmites australis were collected from the Fuxi River basin of Sichuan Province, China.
In April of 2014, the experiment was carried out with hydroponic cultivation in a ventilated plastic shed. Foam boards were placed on the clean plastic barrels with three holes to serve as a plant carrier. Three plants of similar height were selected from each species and transplanted into the foam carriers, and the root of the plants were immersed in the nutrient solution with the modified Hoagland formula: 945 mg/l Ca(NO3)2·4H2O, 506 mg/l KNO3, 80 mg/l NH4NO3, 136 mg/l KH2PO4, 493 mg/l MgSO4, 2.5 ml Fe-EDTA, 5 ml trace element solution (0.83 mg/l KI, 6.2 mg/l H3BO4, 22.3 mg/l MnSO4, 8.6 mg/l ZnSO4, 0.25 mg/l Na2MoO4, 0.025 mg/l CuSO4, 0.025 mg/l CoCl2), pH = 6.0.
After two weeks of continued growth of the plant’s, the Cu(NO3)2 was added to the aqueous solution according to the concentration gradient: 7 concentration process of 0, 10, 25, 60, 100, 200 and 500mg/l. Each of concentration process was repeated six times.
The well-growing plants were collected after three weeks and divided into above- and below-ground parts. They were washed with distilled water and deionized water twice, treated with deactivation enzymes at 105°C, and dried to a constant weight for eight hours at 70°C. After drying, weighing, grinding and digestion, the plant samples were determined Cu2+ concentration by atomic absorption spectrophotometer.
Copper retention rate was calculated by formula (1) (Xia & Shu, 2001).
C1: Cu2+ content in underground part of plants, mg/kg.
C2: Cu2+ content in aboveground part of plants, mg/kg.
The experimental data was analyzed by variance analysis (ANOVA), LSD test, and correlation analysis between Cu2+ concentration of solution and enrichment concentration of plants by Origin 9.0 software.
Results and discussion
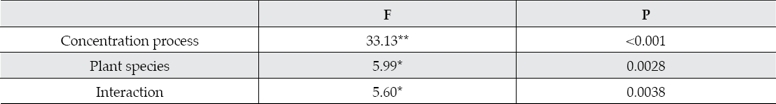
The Two-way ANOVA analysis of Cu2+ contents of the two emergent plants is as follows: The plant species and the Cu2+ concentration process significantly affected the accumulation of copper in plants. The analysis results were showed in Table 1.
Table 1 The ANOVA analysis of Cu2+ accumulation
* Notes: ** indicates a significant difference at p = 0.001; *indicates a significant difference at p = 0.01
Calamus and reeds showed very significant differences at seven concentration processes for the Cu2+ accumulation capacity in different emergent plants. The Cu2+ accumulation content in different plant species (calamus and reeds) has significantly affected (P < 0.01). In addition, the interaction of different Cu2+ concentrations and plant species also significantly affects Cu2+ enrichment capacity of wetland plants (P < 0.01).
Table 2 shows Cu2+ accumulation in Acorus calamus and Phragmites australis.
Table 2 Cu2+ accumulation in Acorus calamus and Phragmites australis.
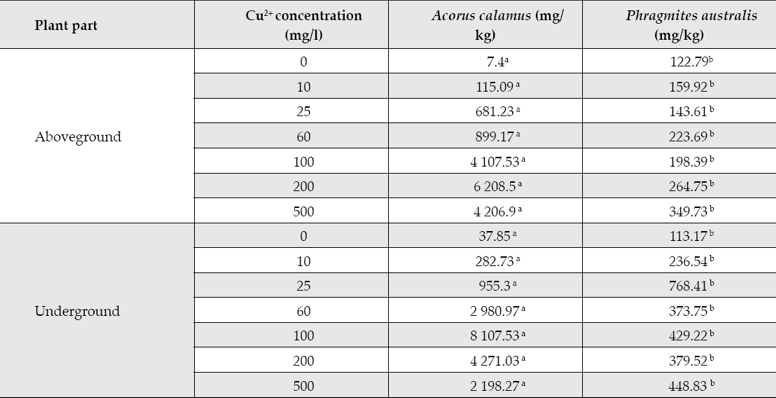
* Notes: Values with different letters in the same element of the same species and same column indicate a significant difference at p = 0.05 according to the LSD test.
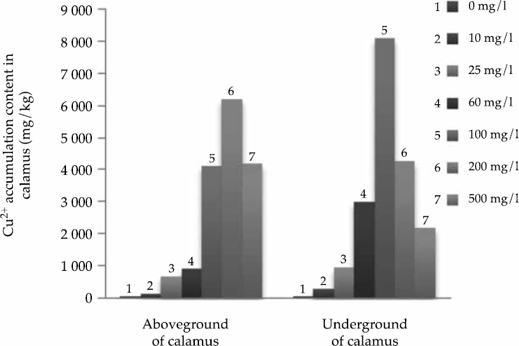
Figure 1a and 1b indicated that the Cu2+ accumulation contents in calamus and reed showed significant differences. In general, Cu2+ accumulation amounts in calamus were significantly more than in the reed. The calamus has a stronger ability to absorb and transport Cu2+ than reed does.
The experiment results showed that the Cu2+ accumulation contents of calamus were significantly more than that of reed in both the above- and below-ground parts. The Cu2+ enrichment amounts in the underground parts of the two plant species were not significantly different at low Cu2+ concentrations (10 mg/l and 25 mg/l). Yet, the Cu2+ enrichment amounts in calamus were significantly more than that in the reed at higher Cu2+ concentrations (60 mg/l, 100 mg/l, 200 mg/l and 500 mg/l). The aboveground parts of plants were similar to belowground parts
Overall, in lower concentrations (10 and 25 mg/l), Cu2+ accumulation quantity in Acorus calamus and Phragmites australis were similar at the 0.05 level. Cu2+ accumulation quantity in the above- and below-ground reed parts were significantly different at higher Cu2+ concentrations, but the same parts of the calamus were not significantly different. The two plant species showed different accumulation abilities at different Cu2+ concentrations; calamus accumulated more Cu2+ than the reeds did, as shown in Figure 1a and 1b.
The accumulation amount of Cu2+ in calamus and reed increased with the increase of pollutant concentration. At medium and low concentrations, the contents in the underground part of calamus were more than that in the aboveground part, and the average retention rate was more than 50%. However, it was the opposite at high concentrations (200 and 500 mg/l); the content in the underground parts was lower. The calamus has a strong ability to enrich and migrate Cu2+. The content of underground part of reed was higher than that of aboveground parts at six concentration processes, indicating that the root system of reed had a strong retention effect on Cu2+, and the average retention rate was more than 40% (Table 3). There was no significant difference in mean retention rate between the two species.
The results of the correlation analysis of Cu2+ contents in the aboveground parts of calamus and hydroponic solution Cu2+ concentration showed that a maximum Cu2+ content was achieved when the concentration was 200mg/l. The variation curve of Cu2+ content in the underground and aboveground parts were similar. The optimum enrichment concentration for copper was 100mg/l (Figure 2).
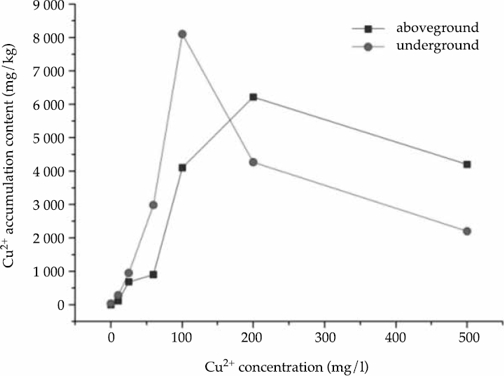
Figure 2 The correlation between Cu2+ accumulation content in Acorus calamus and Cu2+ concentration.
Cu2+ accumulation contents in plants reaches a maximum with the increase of Cu2+ concentration in hydroponic solution. Cu2+ accumulation contents in plants decreased if the concentration exceeded the optimum enrichment concentration, indicating that the toxicity of heavy metals to plants has exceeded its tolerance, inhibiting plant growth and halting the accumulation of Cu2+.
The Cu2+ enrichment amount in the aboveground of reed increased with the increase of Cu2+ concentration in the solution through the correlation analysis curve (Figure 3). Cu2+ accumulation content showed slight fluctuations at low concentration processes (10, 25, 60 and 100 mg/l), but Cu2+ accumulation significantly increase as the hydroponic solution of Cu2+ concentration increased. Therefore, Phragmites australis showed good Cu2+ tolerance. Cu2+ content in the underground parts of reed gently increased as hydroponic solution Cu2+ concentration increased, showing a positive correlation relationship. The enrichment amount of Cu2+ in the underground part was significantly more than that in the aboveground part. As can be seen from Figure 3, Cu2+ accumulation contents in the underground portion of reed fluctuated under low Cu2+ concentrations. As Cu2+ content increased, Cu2+ content stabilized and Cu2+ content reached its maximum in reeds.
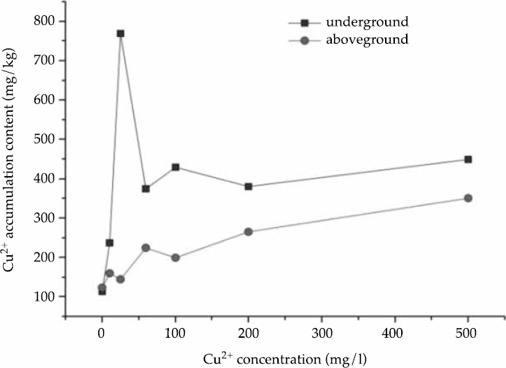
Figure 3 The correlation between Cu2+ accumulation content in Phragmites australis and Cu2+ concentration.
The absorption of copper by calamus was higher than that of reed at seven concentration processes, but reed tolerance to high Cu2+ concentration was higher than calamus. Therefore, calamus is suitable for the ecological restoration of plants for medium and low concentration of heavy metal polluted water bodies.
According to the results of correlation analysis between Cu2+ content in plants and Cu2+ concentration in the hydroponic solution, the Cu2+ accumulation content(y) in aboveground and underground parts of Acorus calamus and Phragmites australis increased as Cu2+ concentration (x) increased; there was a significant positive correlation (Table 4). Thisindicated that the concentration of heavy metals in the growing medium plays an important role that impacts plant absorption and heavy metal accumulation.
Cu2+ primarily accumulates in plant roots (Stolts & Gregor, 2002). This is because Cu2+ in roots is the primarily forms from precipitation. Additionally, there is ionic and complex states of Cu in the plant saps. It is difficult to transport Cu2+ in plant roots due to retention, passivation, or precipitation. Cu2+ accumulates in the root surface mainly in the form of microcrystals on cell walls. Plants accumulated them in the roots, which prevents harmful ions from inhibiting photosynthesis. In this study, Cu2+ accumulation quantities in the underground parts of the two wetland plants were significantly greater than those in the aboveground parts (Ren, Tao, & Yang, 2009).
Conclusions
The two wetland plant species showed strong Cu2+ enrichment abilities at different Cu2+ concentration conditions, both in underground microcrystals on cell walls. ons from and aboveground parts. The contents of Cu2+ in calamus and reed were significantly different at the same Cu2+ concentration. Among same type of wetland plants, while below 200 mg/l, Cu2+ accumulation increased as the Cu2+ concentration in the solutions increased.
Because they have the ability to effectively-absorb and enrich copper, both Acorus calamus and Phragmites australis can be used as selective species for phytoremediation of contaminated water bodies with heavy metal. The Phragmites australis has better tolerance to heavy metals Cu2+, and compared to other plants, the Acorus calamus has stronger absorption and accumulation Cu2+ abilities (Table 5). Overall, Acorus calamus is a better heavy metal contaminated water remediation plant than the Phragmites australis.











 nueva página del texto (beta)
nueva página del texto (beta)