Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Tecnología y ciencias del agua
versión On-line ISSN 2007-2422
Tecnol. cienc. agua vol.3 no.1 Jiutepec ene./mar. 2012
Artículos técnicos
Filtering rates and functional response of selected zooplankton on the bacterium Vibrio cholerae Non O1 Non O139
Tasas de filtración y respuesta funcional de una selección de zooplancton sobre la bacteria Vibrio cholerae No O1 No O139
Pedro Ramírez, Abigail Tovar, S. Nandini and S.S.S. Sarma
Universidad Nacional Autónoma de México.
Institutional direction of the authors
Dr. Pedro Ramírez García
Universidad Nacional Autónoma de México
Facultad de Estudios Superiores Iztacala
Unidad de Investigación Interdisciplinaria en Ciencias de la Salud y la Educación (UIICSE)-CyMA/Laboratorio de Bacteriología
Avenida de Los Barrios 1, Colonia Los Reyes Iztacala
54090 Tlalnepantla, Estado de México, México
Teléfono: +52 (55) 56231 333, extensión 39724
micro@servidor.unam.mx
Biól. Abigail Tovar
Universidad Nacional Autónoma de México
Unidad de Investigación Interdisciplinaria en Ciencias de la Salud y la Educación (UIICSE)-CyMA/Laboratorio de Bacteriología
Avenida de Los Barrios 1, Colonia Los Reyes Iztacala
54090 Tlalnepantla, Estado de México, México
abi0014@hotmail.com
Dra. Nandini Sarma
Universidad Nacional Autónoma de México
UMF/Laboratorio de Zoología Acuática
Avenida de los Barrios 1, Colonia Los Reyes Iztacala
54090 Tlalnepantla, Estado de México, México
Teléfono: +52 (55) 5623 1125
nandini@servidor.unam.mx
Dr. Sri Singaraju Subrahman Sarma
Universidad Nacional Autónoma de México
UMF/Laboratorio de Zoología Acuática
Avenida de Los Barrios 1, Colonia Los Reyes Iztacala
54090 Tlalnepantla, Estado de México, México
Teléfono: +52 (55) 56231 256
sarma@servidor.unam.mx
Received: 30/07/10
Accepted: 4/07/11
Abstract
Vibrio cholerae strains frequently cause cholera outbreaks in tropical countries. It has been suggested that zooplankton play an important role in transmitting cholera from aquatic systems to their human hosts. It is also possible that zooplankton—which are known to have a range of diets, including bacteria, detritus, algae and other zooplankton—could also help to filter out Vibrio from the medium. Here we tested this hypothesis using 11 taxa of rotifers, cladocerans, copepods and ostracods. To determine feeding rates, 10 batches of each taxon were used; they were counted using a stereoscopic microscope and placed into individual test beakers. A control group was also established. An aliquot (10 ml) of the bacterial dilution (10-12) was added to each test beaker with a bacterial concentration (2.5 x 106) that previously contained 50 ml of EPA sterile solution. Grazing time was 30 min, after which 2.5 ml formalin was added to each test tube to stop feeding. Functional response experiments were conducted with different dilutions of V. cholera, ranging from 10-1 to 10-12. The cladocerans, particularly Moina macrocopa, were most efficient at reducing bacterial densities while the rotifers were the least. The data are discussed with emphasis on the role of these zooplankton in controlling V. cholerae in nature.
Keywords: zooplankton, Vibrio cholerae, filtering rates, functional response.
Resumen
La cepas de Vibrio cholerae frecuentemente causan brotes de cólera en los países tropicales. Se ha sugerido que el zooplancton desempeña un papel importante en la transmisión del cólera de los sistemas acuáticos a sus huéspedes humanos. También es posible que el zooplancton, del cual se conoce que se alimenta de diferentes tipos de dietas, incluyendo bacterias, detritus, algas e incluso de otros integrantes del mismo zooplancton, también pudiera ayudar en la eliminación de Vibrio del medio. En este trabajo se probó la hipótesis utilizando 11 taxones de rotíferos cladóceros, copépodos y ostracodos. Para las tasas de alimentación se utilizaron lotes de diez individuos de cada taxón, que se contaron en un microscopio estereoscópico y se colocaron en un vaso de precipitados; también se colocó un control. Una alícuota (10 ml) de la dilución bacteriana (10-12) se añadió a cada vaso de prueba, conteniendo una concentración bacteriana de (2.5 x 106); en los vasos se habían colocado previamente 50 ml de solución EPA estéril. El tiempo de pastoreo fue de treinta minutos, después de lo cual se añadió a cada vaso de prueba 2.5 ml de formol para detener el consumo. Los experimentos de respuesta funcional se llevaron a cabo en diferentes diluciones de V. cholerae en rangos de 10-1 a 10-12. Los cladóceros, particularmente Moina macrocopa, fueron los más eficientes para reducir la densidad bacteriana, mientras los rotíferos fueron los menos. Los datos han sido discutidos con énfasis sobre el papel de este zooplancton para controlar V. cholerae en la naturaleza.
Palabras clave: zooplancton, Vibrio cholerae, tasas de filtración, respuesta funcional.
Introduction
One of the most common water borne diseases in the tropics is cholera, caused by Vibrio cholerae. Strains of Vibrio, commonly found in lakes, rivers, and estuaries are toxic when they enter their hosts (Colwell and Spira, 1992). Toxic and non-toxic strains are known to co-exist in several water bodies. In the coastal states of Mexico several strains have been recorded. Many species of Vibrio use chitin as the source of carbon (Meibom et al., 2004), which is one of the most common polymers in nature. In freshwaters, chitin is found in the carapace of microcrustaceans mainly cladocerans, copepods and ostracods. It has been hypothesized that copepods play an important role in transmitting cholera from the aquatic systems to their human hosts (Rawlings et al., 2007).
Microcrustaceans such as cladocerans, copepods and ostracods, along with rotifers often form the dominant biomass of plankton in freshwaters. Most of these organisms are filter feeders and are capable of consuming bacteria (Dodson and Frey, 2001; Wallace et al., 2006). The pseudotrochal cilia of Brachionus do have the capacity to filter small food particles (Kutikova, 1970). The generalist, non-selective mode of feeding of cladocerans allows their extensive use in lake management for consuming and clearing cyanobacteria (Gulati et al., 1990). The capability of cladocerans to filter bacteria depends largely on the intersetular space between its thoracic limbs. It has been shown that certain genera such as Daphnia are more capable of adjusting their filtering apparatus and the intersetular distance so as to maximize their food intake (Ghadouani and Pinel-Alloul, 2002).
Functional response studies, increasing prey consumption in relation to increasing prey availability, are elegant tools to determine the feeding rates of organisms. Most filter feeders show a Type I trend in functional responses, that is a direct relation in the increase in prey consumption with increasing prey availability (Jeschke et al., 2004). While it is well known that cladocerans can filter and consume bacteria in general, not much is known about these aspects with regard to Vibrio.
The canals of Xochimilco in Mexico City are the final remnants of a large lake that once covered the valley of Mexico (De la Lanza and García, 2002). Unfortunately, natural springs that once filled the lakes and canals have been exploited for drinking water and these water bodies are now filled partially with treated sewage water. Bacterial, including V. cholerae levels are therefore very high in these waters. Here we set out to compare the capability of several zooplankton taxa in consuming the V. cholerae. Here we did not use strains 01 and 0139 which have been known to cause outbreaks of cholera in several Asian and American countries (Estrada-García and Mintz, 1996).
Bacterial contamination of freshwaters is on the rise, particularly in water bodies that receive treated waste waters to maintain their level. In Lake Xochimilco (Mexico City) we have observed high concentrations of the bacterium Vibrio cholerae, probably due to the fact that incompletely treated waste water is used to maintain the water level in the canals. In fresh waters, rotifers, cladocerans and photosynthetic flagellates are significant consumers of bacteria (Sanders and Porter, 1988; Monakov, 2003). Information is scarce on bacterivory by cladocerans in Mexico particularly on their capacity to feed on pathogenic bacteria. Here we compared the relative feeding efficiency of eleven zooplankton taxa, all found in Lake Xochimilco, on V. cholerae isolated from the same lake and cultured on TSBS medium.
Material and methods
The zooplankton taxa used in this study were the rotifers Plationus patulus, Brachionus havanaensis, B. rubens; the cladocerans Alona rectangula, Moina macrocopa, Ceriodaphnia dubia and Daphnia pulex; the copepods Leptodiaptomus sp., Eucyclops serrulatus, Elaphoidella grandidieri and the ostracod Heterocypris incongruens. All the taxa were cloned and maintained on a diet of the green algae Chlorella vulgaris or Scenedesmus acutus at a density of 0.5x106 cells ml-1. The species were cultured on moderately hard water (EPA medium), at a temperature of 23 ± 2°C. The algal species were cultured on Bold medium (Borowitzka and Borowitzka, 1988). The algae were harvested after 8-10 days, allowed to sediment in a refrigerator, decanted and enumerated using a Neubauer Haemocytometer. Cultures were changed twice a week when they were in one liter recipients.
A selected Vibrio cholerae strain Non O1 Non O139 isolated from Xochimilco lake in Mexico City was grown in Luria broth at 37°C. In vitro growth kinetics of V. cholerae strain was determined as follows. An aliquot (50 ml) from the initial culture was transferred to individual flask (A) and was incubated at 37°C on rotary shaker 18 h. The next step was to transfer 2 ml of this culture in 98 ml (B) of Luria broth and incubate it in the same condition; the growth was monitored by observing changes in the optical density at 630 nm over 4h; growth curves were performed in triplicate and the OD630 measurements were averaged for each time to generate the growth curves. Immediately following inoculation an aliquot (0.1 ml) of the culture was removed, serially diluted (10-7 to 10-12), and plated on TSA agar plates for cell enumeration. Once the number of bacteria corresponding to each dilution was determined, grazing experiments were initiated.
Before starting bacterial grazing, rotifers and cladocerans were introduced in EPA sterile solution for 1 hour, under starvation conditions. After that, the zooplankters were rinsed on a membrane 0.4 mm pore opening at low vacuum Millipore equipment and transferred to beaker with EPA solution. Ten individuals of each taxon were counted using a stereoscopic microscope and put it in each test beaker plus control. In all there were 48 recipients (11 taxa + 1 control with four replicates each). An aliquot (10 ml) of the bacterial dilution (10-12) was added to each 10 test beaker with bacterial concentration (2.5 x 106) that previously contained 50 ml of EPA sterile solution. Grazing time was 30 min and past this time 2.5 ml formalin was add to each glass test to stop feeding.
To quantify bacterial consumption, samples (0.5 ml) from each beaker, without zooplankters, were filtered in 0.22 µm polycarbonate membrane and stained with 50 µl 4´6-diamino-2-phenylindole (DAPI) (Porter and Feig, 1980) 5-10 min. Bacteria were counted using fluorescence microscope (Nikon E-600) 100x, grid were counted in a field, consisting of 100 boxes and the counting was done by frame, horizontally. To calculate number of bacteria we applied the following formula:
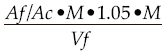
Where:
Aƒ = Filtration area
Ac = Counting unit area (square, line, grill or field)
M = Average of bacteria
1.05 = Correction factor for the formalin
Vƒ= Volume filtered
D = Number of times the sample was diluted
To prepare functional response new dilution series were prepared from 10-1 to 10-12 and selected the interval 10-3 to 10-8. With the results an ANOVA analysis was done to know the significant differences of consumption between different species tested and compared the percentage removal i.e. efficiency of bacterivory by the zooplankters tested.
Results
Our data indicate that although all the rotifers were able to filter Vibrio (figure 1), none of them could decrease it significantly (P > 0.05, one way ANOVA, table 1). Among the micro-crustaceans tested, the cladocerans could filter Vibrio better than the copepods and ostracods. Although the cladocerans were able to significantly reduce the density of the bacteria in relation to the controls (P < 0.05, one way ANOVA, table 1) there were no significant differences with relation to the body size of the test species (table 1). Among the copepods and ostracod too, there was no difference in the ingestion rates in relation to the body size.

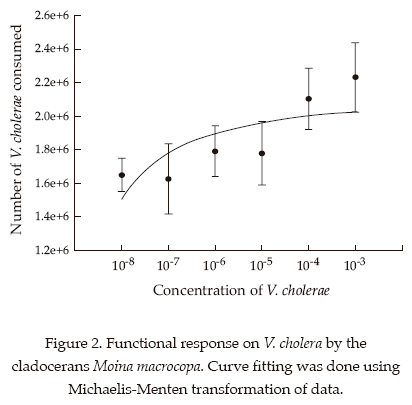
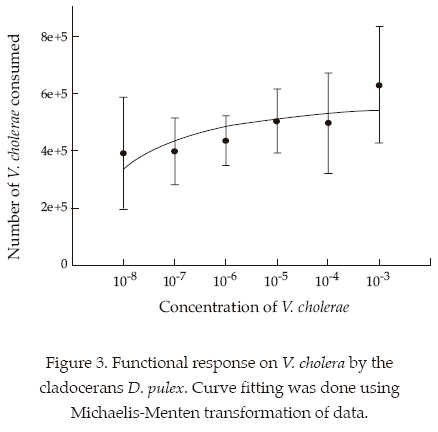
The functional response of the cladocerans Moina macrocopa (figure 2) and Daphnia pulex (figure 3) showed a Type II response in that there was an increase in the number of bacteria consumed with increasing V. cholerae density. Moina was superior to Daphnia in relation to its filtering ability
Discussion
Vibrio cholerae, the bacterium that causes cholera, occupies two distinct habitats: aquatic environments and human intestines. Although its activity in human hosts has received a great deal of attention because of its effect on human health, the microbe's behavior in natural ecosystems has received much less consideration. V. cholerae acts as a typical heterotrophic bacterium, while in aquatic environments mineralizing organic matter for reuse within the food web (Estrada-García and Mintz, 1996). In addition, three key processes affect the microorganism's population dynamics and therefore its role in ecosystem processes: attachment to environmental substrates, and bacterivory.
Several rotifer taxa, but not all, are effective bacterivores. All the rotifers tested here belong to the Brachionidae which is capable of feeding on bacteria, detritus and algae in the size range of 2-20 µm (Monakov, 2003). Nevertheless, we observed that all these species were unable to significantly lower the concentrations of V. cholerae. It has been documented that these rotifers are not true bacteriophages (Monakov, 2003) and are unable to live on a diet of bacteria alone; smaller brachionids such as Anuraeopsis fissa are quite capable of living on and feeding on bacteria (Ooms-Wilms, 1997; Ooms-Wilms et al., 1995).
In this study we analyzed the consumption of Vibrio at a range of densities within the infective levels. The consumption of bacteria was higher at higher bacterial densities which indicate the utility of these cladoceran taxa in controlling high densities of Vibrio in nature. Among the cladocerans tested here we found that bacterivory on V. cholerae was highest by Moina and Daphnia. In terms of biomass, the former is more efficient at clearing bacteria than the latter. This has a significant application since Moina is more abundant and common throughout the year than Daphnia in Xochimilco (Nandini et al., 2005).
Several studies on cladocerans indicate that they show a Type II functional response on algal or cyanobacterial diets (Monakov, 2003). We also found that, both Daphnia pulex and Moina macrocopa showed similar trends on V. cholera. The ingestion rates were greater in Moina macrocopa than in D. pulex. It has been suggested that species such as Moina macrocopa, dominating eutrophic waters (Dumont and Negrea, 2002), often have fine meshes and are better capable of filtering bacteria. The ability of cladocerans to feed on bacteria largely depends on the intersetular space in their thoracic limbs (Brendelberger, 1991). This is not correlated to the body size of the cladoceran; for instance Daphnia magna is longer than Diaphanosoma brachyurum but the latter is more capable of filtering bacteria. This is the reason why correlations between bacterial filtering rates and body size of cladocerans are often not significant; results observed in this study too.
Copepods and ostracods are efficient bacteriopahges (Monakov, 2003 and articles cited therein). It has been well documented that V. cholerae has a high affinity for the carapace of copepods (Colwell and Spira 1992) but not much is known in this regard for cladocerans. In fact, it has also been suggested that grazing pressure could induce the shift from nontoxigenic to toxigenic strains (Lipp et al., 2002). It is also true, however, that the impact of grazing on bacterial community structure is a very poorly studied subject (Jürgens et al., 1994). It remains to be seen whether cladocerans feed preferentially on V. cholerae in the presence of an alternative diet and whether their ability to consume bacteria can offset a high production of Vibrio since it has been documented that this bacterium is capable of showing high growth rates on the chitinous carapace of cladocerans.
Acknowledgements
We thank financial assistance from PAPCA (Project 85; 2008-2009).
References
BOROWITZKA, M.A. and BOROWITZKA, L.J. Micro-algal biotechnology. Cambridge: Cambridge University Press, 1988, 477 pp. [ Links ]
BRENDELBERGER, H. Filter mesh-size of cladocerans predicts retention efficiency for bacteria. Limnology and Oceanography. Vol. 36, 1991, pp. 884-894. [ Links ]
COLWELL, R.R. and SPIRA, W.M. The ecology of Vibrio cholerae. In Current Topics in Infectious Disease. Cholera. Barua, D. and Greenough III, W.B. (editors). New York: Plenum Publishing Corporation, 1992, pp. 107-127. [ Links ]
DE LA LANZA, E.G. y GARCÍA, C.J.L. Lagos y presas de México. México, D.F.: AGT Editor, S.A., 2002, 680 pp. [ Links ]
DODSON, S.I. and FREY, D.G. Cladocera and other branchiopoda. In Ecology and classification of North American Freshwater Invertebrates. Thorp, J.H. and Covich, A.P. (editors), London: Academic Press, 2001, pp. 850-914. [ Links ]
DUMONT, H. and NEGREA, S. Introduction to the Class Branchiopoda. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. The Netherlands: Leyden Backhuys Publishers, 2002, 398 pp. [ Links ]
ESTRADA-GARCÍA, T. and MINTZ, E.D. Cholera: Foodborne transmission and its prevention. European Journal of Epidemiology. Vol. 12, 1996, pp. 461-469. [ Links ]
GULATI, R.D., LAMMENS, E.H.R.R, MEIJER, M.L., and VAN DONK, E. (editors). Biomanipulation. Tool for water management. Hydrobiologia. Vol. 200/201, ISSN00188158, 1990, pp. 1-628. [ Links ]
GHADOUANI, A. and PINEL-ALLOUL, B. Phenotypic plasticity in Daphnia pulicaria as an adaptation to high biomass of colonial and filamentous cyanobacteria: experimental evidence. Journal of Plankton Research. Vol. 24, 2002, pp. 1047-1056. [ Links ]
JESCHKE, J.M., KOPP, M., and TOLLRIAN, R. Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biological Reviews. Vol. 79, 2004, pp. 337-349. [ Links ]
JÜRGENS, K., ARNDT, H., and ROTHHAUPT, K.O. Zooplankton-mediated changes of bacterial community structure. Microbial Ecology. Vol. 27, 1994, pp. 27-42. [ Links ]
KUTIKOVA, L.A. Kolovratki fauny SSSR (The rotifer fauna of USSR). Keys of the fauna of USSR (in Russian). Vol. 104, 1970, 744 pp. [ Links ]
LIPP, E.K., HUQ, A., and COLWELL, R.R. Effects of Global Climate on Infectious Disease: the Cholera Model. Clin. Microbiol. Rev. Vol. 15, 2002, pp. 757-770. [ Links ]
MEIBOM, K.L., LI, X.B., NIELSEN, A.T., WU, C.Y., ROSEMAN, S., and SCHOOLNIK, G.K. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA. Vol. 101, 2004, pp. 2524-2529. [ Links ]
MONAKOV, A.V. Feeding of freshwater invertebrates. Ghent, Belgium: Kenobi Productions, 2003, pp. 373. [ Links ]
NANDINI, S., RAMÍREZ-GARCÍA, P., and SARMA, S.S.S. Seasonal variation in the species diversity of planktonic rotifers from Lake Xochimilco (México). Journal of Freshwater Ecology. Vol. 20, No. 2, 2005, pp. 287-294. [ Links ]
OOMS-WILMS, A.L., POSTEMA, G., and GULATI, R.D. Evaluation of bacterivory of Rotifera based on measurements of in situ ingestion of fluorescent particles, including some comparisons with Cladocera. Journal of Plankton Research. Vol. 17, 1995, pp. 1057-1077. [ Links ]
OOMS-WILMS, A.L. Are bacteria an important food source for rotifers in eutrophic lakes? Journal of Plankton Research. Vol. 19, No. 8, 1997, pp. 1125-1141. [ Links ]
PORTER, K.G. and FEIG, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. Vol. 25, No. 5, 1980, pp. 943-948. [ Links ]
RAWLINGS, T.K., RUIZ, G.M., and COLWELL, R.R. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the Copepods Acartia tonsa and Eurytemora affinis. Applied and Environmental Microbiology. Vol. 73, 2007, pp. 7926-7933. [ Links ]
SANDERS, R.W. and PORTER, K.G. Phagotrophic phytoflagellates. Adv. Microb. Ecol. Vol. 10, 1988, pp. 167-192. [ Links ]
WALLACE, R.L., SNELL, T.W., RICCI, C., and NOGRADY, T. Rotifera Part 1: Biology, Ecology and Systematics. Guides to the identification of the microinvertebrates of the continental waters of the world. The Netherlands: Kenobi Productions Gent/Backhuys, Leyden, 2006, 299 pp. [ Links ]














