Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.14 no.79 México Set./Out. 2023 Epub 06-Out-2023
https://doi.org/10.29298/rmcf.v14i79.1344
Scientific article
Soil seed bank under isolated trees of the Tamaulipan Thorny Scrub
1Universidad Autónoma de Nuevo León, Facultad de Ciencias Forestales. México.
2Instituto Potosino de Investigación Ciencia y Tecnología. División de Ciencias Ambientales. México.
Studying the seed bank makes it possible to interpret the status of disturbed sites, the response to disturbance-driven changes, and the subsequent dynamics of a plant community. Heterogeneity in seed bank formation is influenced by seed dormancy, seed dispersal type, and such landscape components as topography or vegetation. The number of seeds in the soil depends, in part, on the vegetation present, however, in deforested and fragmented landscapes, isolated trees are the only potential reservoirs for vegetation regeneration. In this work, the spatial and temporal variation in abundance, density, and number of germinable seed species on the ground was explored during two years, under five common isolated tree species in open areas of the Tamaulipan Thorny Scrub. Seed bank characteristics were calculated for each isolated tree species and compared between species, seasons, and years of collection. The seed bank under the canopy of two zoochorous trees (Neltuma laevigata and Diospyros texana) was richer and denser than under the other three species (Yucca filifera, zoochorous; Parkinsonia aculeata and Vachellia farnesiana, unassisted dispersal). Also, more species and seeds germinated in Fall than in Spring, and more herbaceous than arboreal species were recorded.
Key words Diospyros texana Scheele; Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose; regeneration; richness; dispersal syndrome; zoochory
Estudiar el banco de semillas permite interpretar el estado de sitios perturbados, la respuesta a cambios impulsados por disturbios y la consecuente dinámica de una comunidad vegetal. La heterogeneidad en la formación del banco de semillas está influida por la latencia de estas, su tipo de dispersión y componentes del paisaje como topografía o vegetación. El número de semillas en el suelo depende, en parte, de la vegetación presente, sin embargo, en paisajes deforestados y fragmentados, los árboles aislados representan los únicos reservorios potenciales para la regeneración de la vegetación. En este trabajo se exploró, durante dos años, la variación espacial y temporal en la abundancia, densidad y número de especies de semillas germinables en el suelo bajo cinco especies arbóreas aisladas comunes en áreas abiertas del Matorral Espinoso Tamaulipeco. Las características del banco de semillas se calcularon para cada especie de árbol aislado y se compararon entre ellas, estaciones y año de colecta. El banco de semillas bajo las copas de dos árboles zoócoros (Neltuma laevigata y Diospyros texana) fue más rico y denso que bajo otras tres especies (Yucca filifera, zoócora; Parkinsonia aculeata y Vachellia farnesiana dispersión no asistida). También se registraron más especies y semillas germinadas en otoño que en primavera, y más herbáceas que arbóreas.
Palabras clave Diospyros texana Scheele; Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose; regeneración; riqueza; síndrome de dispersión; zoocoria
Introduction
The soil seed bank plays an essential role in plant dynamics; it constitutes an important stage of regeneration, as it stores the plant species that will potentially become established in the soil (De Souza et al., 2006). Seed banks are dynamic in space and time (Fenner and Thompson, 2005); in space, fruits and seeds are dispersed when they fall to the ground or by several vectors such as wind, water or animals (Pijl, 1969). Over time, seeds may remain dormant in the soil, that is, they delay germination due to physical or physiological characteristics in their structures, or simply because the right environmental conditions for germination are not present (Baskin and Baskin, 2004). The seeds reach the soil through seed dispersal and rainfall, and they are eliminated from the soil through germination, predation, and death by pathogens (Fenner and Thompson, 2005).
Soil seed banks may contain species present in the vegetation or that were present before a disturbance and in nearby areas (Bossuyt and Honnay, 2008), and therefore are an important resource for the restoration and resilience of a damaged plant community (Shiferaw et al., 2018). Traditionally, in disturbed communities, degradation is assessed only by the status of the standing vegetation, often forgetting that the analysis of seeds stored in the soil provides crucial information for understanding plant dynamics, interpreting the conservation status, and determining the potential for plant recovery (Ma et al., 2021).
Research on the temporal and spatial variations of the seed bank is approached by studying its basic characteristics such as density, diversity, or richness. An important aspect that influences the spatial distribution of seeds is their dispersal mechanism, since it is through this process that plant populations grow, exchange genes, and survive environmental changes (Levin and Muller-Landau, 2000). For example, seeds dispersed by animals are more frequently deposited near trees with similar means of dispersal than those with different dispersal mechanisms (Saatkamp et al., 2013). This is due to the fact that plants with fruits attractive to animals tend to be visited by these for feeding, protection, nesting, or perching (in the case of birds), and while performing these activities, they defecate and thereby deposit the seeds under the tree canopy (DeMars et al., 2010).
Isolated trees that remain standing after a disturbance or are intentionally left in deforested areas are widely studied and utilized for their influence on the dynamics of regeneration and ecological restoration (Guevara et al., 2005). In addition, if these species attract frugivorous animals, they tend to act as seed reservoirs, increasing the interactions with other plants and animals and thereby maintaining high biodiversity (Camargo et al., 2020). This is likely to occur more frequently in forests and jungles because there are more zoochorous species there than in other ecosystems (Gentry, 1982; Willson et al., 1990), however, in disturbed areas of arid zones, zoochorous dispersal and seed accumulation under isolated trees also occurs (Filazzola et al., 2019), and its study depends on whether it is a useful tool for the recovery of vegetation and the interactions that are lost with the continuous change of land use.
The Tamaulipan Thorny Scrub is located in a semi-arid zone in northeastern Mexico. It is a very fragmented vegetation type, composed of a matrix of induced vegetation (for livestock or agriculture) with small remnant patches of native vegetation, as well as isolated trees of different species (Molina-Guerra et al., 2013). In the thorny scrub, 37 species are recognized as having zoochorous dispersal, which represents a higher percentage of taxa with this type of dispersal compared to other semiarid zones (Jurado et al., 2001a). However, the relationships between plants and dispersers, as well as the influence of the type of dispersal on regeneration, have been scarcely studied in this ecosystem.
Different methods have been used for seed bank research in the Tamaulipan Thorny Scrub (Pando-Moreno et al., 2010; Martínez-Adriano et al., 2021; Valdes-Alameda et al., 2021); seed banks are known to occur within small undisturbed vegetation areas and at their edges, but there is no information on seed banks in cattle ranching sites, the most common land use in the region. On the other hand, due to the fragmentation of the scrubland, the role of isolated trees has been studied in the context of livestock production or agroforestry (Sarmiento-Muñoz et al., 2019), while in the ecological context, seed removal and fruit production were studied in order to compare them with those produced in the remaining continuous vegetation (Jurado et al., 2006; Cuéllar-Rodríguez and Jurado, 2016).
The characteristics of the seed bank were determined in terms of richness and diversity, under isolated individuals of tree species with different dispersal syndromes (classified by the characteristics of their fruits). The soil seed bank was compared under the native species Diospyros texana Scheele and Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose (previously Prosopis laevigata (Humb. & Bonpl. ex Willd.) M. C. Johnst.), Yucca filifera Chabaud (of zoochorous dispersion), Parkinsonia aculeata L., and Vachellia farnesiana (L.) Wight & Arn. (previously Acacia farnesiana (L.) Willd.) (without apparent dispersion syndrome). The temporal variation of the seed bank studied was also explored. The hypotheses were: (1) Isolated individuals of zoochorously dispersed species have a greater richness and density of seeds under their canopies than species with other types of dispersal, due to a greater seed deposition generated by the visit of dispersers that are attracted to the zoochorous fruits, and (2) The composition of the banks is variable between seasons because the plants in the community do not all produce seeds in the same season.
Materials and Methods
Study area
The study area is located within the facilities of the Graduate School of Forest Sciences of the Universidad Autónoma de Nuevo León, in Linares municipality, state of Nuevo León. It lies between the 24°48'2.81" N and 99°31'54.51" W to the north, 24°47'50.17" N and 99°32'10.16" W to the south, 24°47'50.80" N and 99°31'39.01" W to the east, and 24°47'59.27" N and 99°32'18.12" W to the west, and has an average altitude of 379 m. The climate in the area is semi-warm sub-humid with summer rains, described as Bs hw(w) in Köppen’s classification modified by García (2004). Rainfall is irregular from May to June and from September to October, with two dry periods: a short one in summer and a long one in winter (Foroughbakhch et al., 2013). The average annual temperature is 23.7 °C and the average rainfall is 810 mm (Martínez-Adriano et al., 2021). The area is 40 ha in size and consists of rotating cattle grazing plots, with isolated trees of different species that correspond to the vegetation prior to the clearing and that are present in the surrounding areas: the Tamaulipan Thorny Scrub.
Cattle ranching significantly reduces the abundance, dominance and diversity of tree and shrub communities in this scrubland (Mora et al., 2013) in which around 160 species have been counted (Foroughbakhch et al., 2013); although not all have been classified by dispersal syndrome, there are six taxa with explosive fruits (autochorous), 37 with adaptations for animal dispersal (zoochorous), 31 with wind dispersal characteristics (anemochorous), and 43 species with no characteristics clearly associated with any dispersal vector (unassisted) (Jurado et al., 2001a).
The phenology of the species has been studied in a limited number, but the tendency is for the dispersal season to occur around June and July (early summer), or October-November (late fall and early winter), with some taxa bearing fruit in both seasons (García, 1997). In this region, the seeds of some species can germinate above their current distribution range, so they may have the capacity to move to higher altitudes due to climate change (Pérez-Domínguez et al., 2013).
Species selection and sampling
The selected species were mesquite (Neltuma laevigata), white chapote (Diospyros texana), and yuca (Yucca filifera), all of which have zoochorous dispersion, as well as huizache (Vachellia farnesiana) and mexican palo verde (Parkinsonia aculeata), whose fruit characteristics classify them as unassisted because they lack apparent traits related to a dispersal syndrome (Jurado et al., 2001a). The criteria for selecting 10 individuals of each species were as follows: (1) Not having any other adult or fruiting tree within a 10 m radius around it; (2) An average canopy size of 6 m (±1.0), with the exception of cassava which was selected with a canopy size of 10 (±1.0) m, and (3) Being located at less than 90 m (±10) away from the closest continuous fragment of scrubland.
Four samples per tree (40 per species) were collected during the two years of study to evaluate the seed bank under the canopy of each selected tree. The collection period was twice a year, in March and September (early spring and fall), 2021 and 2022, which correspond to the seasons after the dispersal of most of the shrubs and before the germination season.
The collection site was determined at an average distance between the trunk and the top of each individual. The soil sample size was 30×30 cm and 3 cm deep (approximately 1 600±132 g).
Emergence and species identification
The seed bank was estimated using the seedling-emergence method, which is the most useful method for describing the reserves of the entire plant community (Piudo and Cavero, 2005). Soil samples were placed in trays for germination in the greenhouse of the School of Forestry Sciences of the Universidad Autónoma de Nuevo León; they were kept under natural light with a 50 % shade net and at a temperature of 20 to 25 °C. Irrigation was applied every other day in order to maintain constant humidity. Every day, the number and species of all seedlings that emerged during the experiment over a four-month period (when no new seedlings germinated for 15 days) was recorded, and their identification was carried out according to the Texas vascular plant manual (Correll and Johnston, 1970). The identified seedlings were removed from the containers, and the unrecognized seedlings were transplanted into nursery bags in order to allow their growth and subsequent identification. When it was not possible to assign the name of the species, the seedlings were identified at genus level. The emergence of a seedling was regarded as indicative of a viable seed. Once the seedlings were identified, information on the origin, habit, and type of dispersal of the species was obtained (Table 1). The number of emerged seedlings was recorded, without knowing how much time the seeds had spent in the soil.
Table 1 Seed bank species composition, growth habit, origin, and assigned dispersal syndrome.
| Species | Habit | Origin | Dispersal syndrome | |
|---|---|---|---|---|
| Scientific name | Common name | |||
| Acanthaceae | ||||
| Ruellia nudiflora (Engelm. & A. Gray) Urb. | Ruellia | Herbaceous | Native | Explosive fruit (Vargas-Mendoza et al., 2015) |
| Amaranthaceae | ||||
| Amaranthus palmeri S. Watson | Herbaceous | Native | Unassisted (Jurado et al., 2001a) | |
| Amaranthus viridis L. | Herbaceous | Exotic | Anemochorous/ Hydrochorous (SER and RBGK, 2023) | |
| Chenopodium album L. | Quelite | Herbaceous | Unassisted (Williams, 1963) | |
| Apiaceae | ||||
| Cyclospermum leptophyllum (Pers.) Sprague ex Britton & P. Wilson | Apio silvestre | Herbaceous | Native | Anemochorous (Ogle, 2023) |
| Asparagaceae | ||||
| Yucca filifera Chabaud | Yuca | Tree | Native | Zoochorous (Waitman et al., 2012)* |
| Asteraceae | ||||
| Calyptocarpus vialis Less. | Hierba de caballo | Herbaceous | Native | Unassisted (Valerio and Moreira, 1986) |
| Cirsium texanum Buckley | Cardo | Herbaceous | Native | Anemochorous (SER and RBGK, 2023) |
| Conyza canadensis (L.) Cronquist | Herbaceous | Prob. native | Anemochorous (SER and RBGK, 2023) | |
| Erigeron L. sp. | Herbaceous | Anemochorous (Pijl, 1969)* | ||
| Helianthus annuus L. | Girasol | Herbaceous | Native | Unassisted |
| Parthenium confertum A. Gray | Herbaceous | Native | Anemochorous (Mao et al., 2019) | |
| Sanvitalia ocymoides DC. | Herbaceous | Native | Anemochorous (Jurado et al., 2001a) | |
| Sonchus oleraceus L. | Lechuguilla común | Herbaceous | Exotic | Anemochorous (SER and RBGK, 2023) |
| Taraxacum officinale F. H. Wigg. | Diente de león | Herbaceous | Anemochorous (SER and RBGK, 2023) | |
| Boraginaceae | ||||
| Lithospermum matamorense DC. | Herbaceous | Native | Unassisted (Pijl, 1969)* | |
| Brassicaceae | ||||
| Lepidium virginicum L. | Lenteja de campo | Herbaceous | Native | Anemochorous (Díaz and Ríos, 2017) |
| Cactaceae | ||||
| Opuntia Mill. sp. | Tree | Tree | Native | Zoochorous (SER and RBGK, 2023) |
| Campanulaceae | ||||
| Lobelia L. sp. | Herbaceous | Anemochorous (SER and RBGK, 2023) | ||
| Cannabaceae | ||||
| Celtis laevigata Willd. | Palo blanco | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Celtis pallida Torr. | Granjeno | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Convolvulaceae | ||||
| Convolvulus arvensis L. | Herbaceous | Exotic | Unassisted (SER and RBGK, 2023) | |
| Ipomoea L. sp. | Herbaceous | Unassisted (SER and RBGK, 2023) | ||
| Cordiaceae | ||||
| Cordia boissieri A. DC. | Anacahuita | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Ebenaceae | ||||
| Diospyros texana Scheele | Chapote blanco | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Euphorbiaceae | ||||
| Argythamnia humilis (Engelm. & A. Gray) Müll. Arg. | Herbaceous | Native | Unassisted (SER and RBGK, 2023) | |
| Croton ciliatoglandulifer Ortega | Croton | Shrub | Native | Zoochorous (Jurado et al., 2001a) |
| Croton cortesianus Kunth | Croton | Herbaceous | Native | Zoochorous (Jurado et al., 2001a) |
| Croton fruticulosus Engelm. ex Torr. | Croton | Shrub | Native | Zoochorous (Jurado et al., 2001a) |
| Croton incanus Kunth | Croton | Shrub | Native | Zoochorous (Jurado et al., 2001a) |
| Euphorbia prostrata Aiton | Hierba de la golondrina | Herbaceous | Native | Unassisted (Baiges et al., 1991) |
| Tragia ramosa Torr. | Ortiguilla | Herbaceous | Native | Unassisted (Everitt et al, 1999; SER and RBGK, 2023) |
| Fabaceae | ||||
| Desmanthus virgatus (L.) Willd. | Herbaceous | Native | Unassisted (Jurado et al., 2001a) | |
| Galactia texana (Scheele) A. Gray | Herbaceous | Native | Explosive fruit (Jurado et al., 2001a) | |
| Mimosa monancistra Benth. | Uña de gato | Shrub | Native | Unassisted (Jurado et al., 2001a) |
| Neltuma laevigata (Humb. & Bonpl. ex Willd.) Britton & Rose | Mesquite | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Parkinsonia aculeata L. | Mexican palo verde | Tree | Native | Hydrochorous (SER and RBGK, 2023) |
| Vachellia farnesiana (L.) Wight & Arn. | Huizache | Tree | Native | Unassisted (Jurado et al., 2001a) |
| Lamiaceae | ||||
| Teucrium cubense Jacq. | Herbaceous | Native | Unassisted (Jurado et al., 2001a) | |
| Malvaceae | ||||
| Malvastrum coromandelianum (L.) Garcke | Malva | Herbaceous | Prob. native | Unassisted (Jurado et al., 2001a) |
| Sida rhombifolia L. | Herbaceous | Zoochorous (Mori and Brown, 1998) | ||
| Namaceae | ||||
| Nama jamaicensis L. | Herbaceous | Native | Unassisted (Pijl, 1969)* | |
| Onagraceae | ||||
| Oenothera speciosa Nutt. | Hierba del golpe | Herbaceous | Native | Anemochorous (SER and RBGK, 2023) |
| Oxalidaceae | ||||
| Oxalis L. sp. | Herbaceous | Native | Explosive fruit (Unison and ASU, 2023) | |
| Plantaginaceae | ||||
| Plantago lanceolata L. | Plantago | Herbaceous | Exotic | Anemochorous (SER and RBGK, 2023) |
| Portulacaceae | ||||
| Portulaca L. sp. | Verdolaga | Herbaceous | Exotic | Unassisted (SER and RBGK, 2023) |
| Rhamnaceae | ||||
| Condalia hookeri M. C. Johnst. | Brasil | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Rubiaceae | ||||
| Diodia teres Walter | Herbaceous | Native | Unassisted (SER and RBGK, 2023) | |
| Randia rhagocarpa Standl. | Cruceto | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Rutaceae | ||||
| Zanthoxylum fagara (L.) Sarg. | Colima | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Sapotaceae | ||||
| Sideroxylon celastrinum (Kunth) T. D. Penn. | Coma | Tree | Native | Zoochorous (Jurado et al., 2001a) |
| Solanaceae | ||||
| Capsicum annuum L. var. glabriusculum (Dunal) Heiser & Pickersgill | Chile piquín | Shrub | Native | Zoochorous (Jurado et al., 2001a) |
| Physalis viscosa L. | Tomatillo | Shrub | Native | Zoochorous (Jurado et al., 2001a) |
| Urticaceae | ||||
| Urtica chamaedryoides Pursh | Ortiguilla | Herbaceous | Native | Unassisted (Pijl, 1969)* |
| Verbenaceae | ||||
| Glandularia bipinnatifida (Nutt.) Nutt. | Alfombrilla | Herbaceous | Native | Anemochorous (Pijl, 1969)* |
| Lantana L. sp. | Lantana | Shrub | Native | Zoochorous (SER and RBGK, 2023) |
| Verbena canescens Kunth | Verbena | Herbaceous | Native | Anemochorous (Jurado et al., 2001a) |
| Violaceae | ||||
| Hybanthus verticillatus (Ortega) Baill. | Shrub | Unassisted (Pijl, 1969)* | ||
* Personal observation based on the reference; Prob. native = Probably native.
Statistical analysis
The data obtained were used to calculate the richness and density of seedlings per square meter (seedlings m2), as well as the richness and density of herbaceous and tree species. The density is given in square meters in order to facilitate its contrast with other works. Each parameter was compared between the two study years, the seasons of collection (spring vs fall), and isolated tree species.
Given that the data did not meet the assumption of normality (P<0.01), a comparison of medians of richness and density was made with the nonparametric Kruskall-Wallis test and the Bonferroni correction method (for comparison between species), and the Mann-Whitney U test (for comparison between seasons and years) (Zar, 1999). Analyses were performed with SPSS statistics (version 25.0) (IBM, 2022).
Results
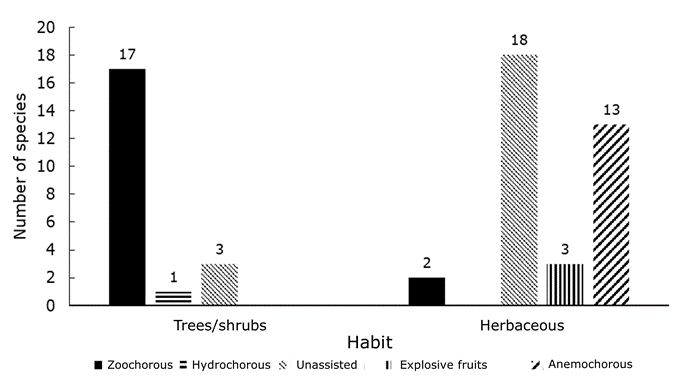
A total of 57 species belonging to 29 families were identified in the seed bank (Table 1): 21 were arboreal or shrubby, and 36 were herbaceous (Figure 1). Of the total number of species, 21 were classified as having unassisted dispersal, 19 as zoochorous, 14 as anemochorous, three as having explosive dispersal or autochorous, and one species as hydrochorous or water-dispersed (Jurado et al., 2001a). Most taxa (45) were considered native or probably native, and five were exotic; the most abundant taxa during the two years of study were Malvastrum coromandelianum (L.) Garcke (754 seedlings total) and Nama jamaicensis L. (616 seedlings total), both herbaceous. The most abundant tree species were Neltuma laevigata (54 seedlings total) and Yucca filifera (49 seedlings total), mostly under the parent plant, and the most abundant seedlings found under a different species were Opuntia Mill. sp. (44 seedlings) and Zanthoxylum fagara (L.) Sarg. (39 seedlings). The best represented families were Asteraceae (9) and Euphorbiaceae (7), followed by Fabaceae (6).
Based on the analysis of the soil seed bank under each isolated tree species, it was determined that seedling richness and density were similar between the two study years (Richness N. laevigata: U=186, Z=-0.38, P=0.71; V. farnesiana: U=194, Z=-0.79, P=0.88; D. texana: U=134, Z=-1.79, P=0.76; Y. filifera: U=135, Z=-1.78, P=0.81; P. aculeata: U=192, Z=0.22, P=0.84; Density N. laevigata: U=197, Z=-0.08, P=0.97; V. farnesiana: U=180, Z=-0.58, P=0.60; D. texana: U=147, Z=-1.43, P=0.15; Y. filifera: U=184, Z=-0.42, P=0.67, P. aculeata: U=147, Z=-1.43, P=0.15).
The comparison between collecting seasons resulted in a higher richness and density of seedlings in the autumn bank under all species (Richness N. laevigata: U=49, Z=-4.12, P<0.01; V. farnesiana: U=86, Z=-3.10, P=0.002; D. texana: U=80, Z=-3.27, P=0.001; Y. filifera: U=60, Z=-3.82, P<0.01; P. aculeata: U=64, Z=-3.77, P<0.01; Density N. laevigata: U=40, Z=-4.13, P<0.01; V. farnesiana: U=80, Z=-3.24, P=0.001; D. texana: U=132, Z=-1.82, P=0.06; Y. filifera: U=74, Z=-3.42, P<0.01; P. aculeata: U=63, Z=-3.71, P<0.01).
The zoochorous dispersal taxa (N. laevigata and D. texana), had a higher average canopy richness than the rest of the species (H=21.14; d. f.=4; P<0.001). In addition, the seedling density was higher under N. laevigata than under V. farnesiana and P. aculeata; the number of species with unassisted seeds was even larger under N. laevigata with respect to Y. filifera, which is also zoochorous (H=23.44; d. f.=4; P<0.001.) (Figure 2).
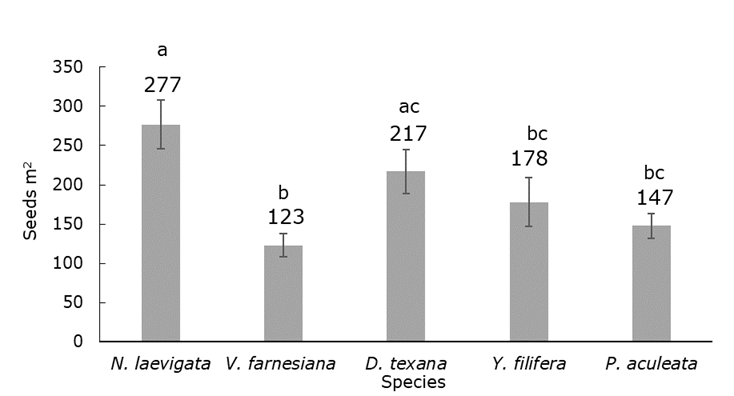
Density data are rounded to the nearest whole number. The error bars represent the standard deviation.
Figure 2 Average seedling density under each species.
In general, more richness and density of herbaceous than of arboreal species was obtained under all species (Richness U=3 765, Z=-3.11; P=0.002; Density U=4 075, Z=-2.31, P=0.02). More tree seedlings emerged on average under D. texana and Y. filifera (two species, H=29.93, d. f.=4, P<0.001), and more herbaceous species under N. laevigata (five species, H=13.96, d. f.=4, P=0.007). The highest density of arboreal species seedlings was found to occur under N. laevigata, D. texana, and Y. filifera, while the density of herbaceous plants was highest under N. laevigata (H=22.47, d. f.=4, P<0.001; H=16.97, d. f.=4, P=0.002, respectively) (Figure 3).
Discussion
The results of this study showed a higher species richness in the seed bank than in any other study conducted in the Tamaulipan Thorny Scrub; 27 more species were identified than those recorded by Martínez-Adriano et al. (2021), who cite 30 species in seed banks present in remnant fragments of the Tamaulipan Thorny Scrub. Likewise, the richness was higher than that estimated in an area of dense scrub (33 species) (Pando-Moreno et al., 2010). On the other hand, the average densities of seedlings m2 under N. laevigata and V. farnesiana are between those recorded in the dense area (1 900 seeds m2) and in an eroded area (62 seeds m2), both with Tamaulipan Thorny Scrub (Pando-Moreno et al., 2010).
There was no annual variability in seed bank density and richness, but there was seasonal variation, as there were more species and density in the seed bank collected in autumn. In other semi-arid regions, variations in species richness, abundance, and seed density in the soil are recorded in different years, due to variations caused by extreme climatic conditions (Quevedo-Robledo et al., 2010; Dreber and Esler, 2011; da Silva et al., 2013). Fruit production can change depending on environmental factors and on the resources that are variable over time, consequently, temporal changes are observed in seed banks (Quevedo-Robledo et al., 2010); although some plants are very constant and maintain their phenological patterns or respond to the variability of resources with a shift of a few days in their phenological stages (Gordo and Sanz, 2009). Slight variation in fruit production may not be sufficient to be reflected in the seed bank composition in short-term studies.
On the other hand, in zoochorous plants, the abundance of fruit consumers may increase or reduce the density of deposited seeds (García and Martínez, 2012), however, there is little or no information on these interactions in the region. Furthermore, the various species in a community usually have different germination strategies, and with the method used in this research, these strategies directly influence the results, so that the recorded seasonal variation may depend on whether the necessary germination conditions are met or not. For the plants of the thorny scrub, Jurado et al. (2001b) indicated that some of the studied species germinated more in autumn, consistently with the results documented herein. Seed bank research using other methods such as sieving, may help to confirm whether there are differences in seed numbers or whether the seasonal variations detected are caused by the germination adaptations of the community. The analysis of the seed bank must take into account this seasonal variation, since sampling in a single season would provide incomplete information for the proper evaluation of disturbed areas or the determination of the potential plant richness.
The results show that zoochorous plants had more species and seed density under their canopies, consistently with the stated hypothesis. Similar results have been described for other arid, semi-arid, and dry forests (Warnock et al., 2007; Hadinezhad et al., 2021), as well as for tropical (Camargo et al., 2020), Mediterranean (González‐Varo et al., 2017), and temperate ecosystems (Rodríguez‐Pérez et al., 2014).
Most of the seeds of the identified tree species have zoochorous dispersal characteristics and are found under a zoochorous tree species (D. texana); thus, the dispersing fauna, among other factors, may shape the tree community as is the case in other ecosystems (Warnock et al., 2007; Hadinezhad et al., 2021). Moreover, seed removal is slower under isolated trees than in the dense thornscrub (Jurado et al., 2006), which may also influence the number of species identified. According to Jurado et al. (2006), the removal was slower, especially in isolated N. laevigata trees, this is one of the species with the highest richness and density of seeds in the studied Tamaulipan Thorny Scrub. The same removal process may determine the seed composition of isolated chapote individuals, which was similar to that of mesquite. The above seems to indicate that in this semi-arid region, the choice of tree species to leave standing or for reforesting influences the ability of the seeds to reach disturbed sites and remain in the seed bank.
The largest number of herbaceous species was obtained in the seed bank under N. laevigata, with a high density and anemochorous or unassisted dispersal. Likewise, the herbaceous species outnumbered the tree species under all species. This is related to the fact that herbaceous plants tend to produce many small seeds, whereas trees produce fewer but larger seeds (Moles et al., 2004; Moles et al., 2005), also, they are selected more often by predators (Dylewski et al., 2020), a fact which may have influenced the recorded lower density. Another factor is that in general, more herbaceous than woody species are dormant in ecosystems with extreme conditions such as those with frost or drought, as is the case of the Tamaulipan Thorny Scrub. The ratio of dormant to non-dormant species does not differ significantly (Jurado and Flores, 2005).
All this information is important because it represents the potential richness that would be obtained in a natural regeneration trajectory. With the evaluation of seed banks and standing vegetation, it is possible to interpret whether areas that appear degraded will recover the pre-disturbance vegetation structure and diversity or have an alternative status (Ma et al., 2021). In the present study, a high density and richness of native species was determined, in addition, it is inferred that there is an influx of seeds from nearby areas, which suggests that the studied area is in a suitable condition to recover its vegetation through natural regeneration, once disturbances cease to occur.
Conclusions
Isolated trees of the native species Diospyros texana, Neltuma laevigata, Yucca filifera, Parkinsonia aculeata and Vachellia farnesiana of the Tamaulipan Thorny Scrub, are reservoirs of a high richness and diversity of seeds. Seed banks composed under species with zoochorous dispersal maintain a higher seed richness and density than those consisting under taxa with other dispersal syndromes. The number of species and the density of germinable seeds undergo a seasonal variation, as the assessed parameters are higher in autumn than in spring.
Acknowledgments
The authors are grateful to PAYCYT-UANL and Conahcyt (626203), and to Ángel López, Izamar Aguirre, and David González for their support in fieldwork.
REFERENCES
Baiges, J. C., X. Espadaler and C. Blanché. 1991. Seed dispersal in W Mediterranean Euphorbia species. Botanika Chronika 10:697-705. https://www.researchgate.net/publication/257933870_Seed_dispersal_in_West_Mediterranean_Euphorbia_L . (31 de julio de 2023). [ Links ]
Baskin, J. M. and C. C. Baskin. 2004. A classification system for seed dormancy. Seed Science Research 14(1):1-16. Doi: 10.1079/SSR2003150. [ Links ]
Bossuyt, B. and O. Honnay. 2008. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. Journal of Vegetation Science 19(6):875-884. Doi: 10.3170/2008-8-18462. [ Links ]
Camargo, P. H. S. A., M. A. Pizo, P. H. S. Brancalion and T. A. Carlo. 2020. Fruit traits of pioneer trees structure seed dispersal across distances on tropical deforested landscapes: Implications for restoration. Journal of Applied Ecology 57(12):2329-2339. Doi: 10.1111/1365-2664.13697. [ Links ]
Correll, D. S. and M. C. Johnston. 1970. Manual of the vascular plants of Texas. Texas Research Foundation. Rener, TX, United States of America. 1881 p. [ Links ]
Cuéllar-Rodríguez, G. and E. Jurado. 2016. Seeds and seedlings from isolated mesquite trees. The Journal of the Torrey Botanical Society 144(1):58-62. Doi: 10.3159/TORREY-D-15-00070.1. [ Links ]
da Silva, K. A., D. M. dos Santos, J. M. F. F. dos Santos, U. P. de Albuquerque, E. M. N. Ferraz and E. L. Araújo. 2013. Spatio-temporal variation in a seed bank of a semi-arid region in northeastern Brazil. Acta Oecologica 46:25-32. Doi: 10.1016/j.actao.2012.10.008. [ Links ]
De Souza M., M., F. C. Maia y M. A. Pérez. 2006. Bancos de semillas en el suelo. Agriscientia 23(1):33-44. http://www.scielo.org.ar/pdf/agrisc/v23n1/v23n1a05.pdf . (31 de julio de 2023). [ Links ]
DeMars, C. A., D. K. Rosenberg and J. B. Fontaine. 2010. Multi-scale factors affecting bird use of isolated remnant oak trees in agro-ecosystems. Biological Conservation 143(6):1485-1492. Doi: 10.1016/j.biocon.2010.03.029. [ Links ]
Díaz S., L. y C. Ríos A. 2017. Diásporas de las arvenses más agresivas en los agroecosistemas de Cuba. Centro Agrícola 44(2):75-82. http://scielo.sld.cu/scielo.php?pid=S0253-57852017000200010&script=sci_arttext . (31 de julio de 2023). [ Links ]
Dreber, N. and K. J. Esler. 2011. Spatio-temporal variation in soil seed banks under contrasting grazing regimes following low and high seasonal rainfall in arid Namibia. Journal of Arid Environments 75(2):174-184. Doi: 10.1016/j.jaridenv.2010.09.007. [ Links ]
Dylewski, Ł., Y. K. Ortega, M. Bogdziewicz and D. E. Pearson. 2020. Seed size predicts global effects of small mammal seed predation on plant recruitment. Ecology Letters 23(6):1024-1033. Doi: 10.1111/ele.13499. [ Links ]
Everitt, J. H., D. L. Drawe and R. I. Lonard. 1999. Field guide to the broad-leaved herbaceous plants of South Texas: used by livestock and wildlife. Texas Tech University Press. Lubbock, TX, United States of America. 277 pp. https://www.ttupress.org/9780896724006/field-guide-to-the-broad-leaved-herbaceous-plants-of-south-texas/ . (31 de julio de 2023). [ Links ]
Fenner, M. and K. Thompson. 2005. The ecology of seeds. Cambridge University Press. Cambridge, CB, United Kingdom. 260 p. [ Links ]
Filazzola, A., A. R. Liczner, M. Westphal and C. J. Lortie. 2019. Shrubs indirectly increase desert seedbanks through facilitation of the plant community. PLoS One 14(4):e0215988. Doi: 10.1371/journal.pone.0215988. [ Links ]
Foroughbakhch, R., M. A. Alvarado-Vázquez, A. Carrillo P., J. L. Hernández-Piñero and M. A. Guzmán L. 2013. Floristic diversity of a shrubland in northeastern Mexico. Phyton International Journal of Experimental Botany 82:175-84. http://www.scielo.org.ar/pdf/phyton/v82n2/v82n2a04.pdf . (4 de mayo de 2023). [ Links ]
García A., L. C. 1997. Estudio fenológico y de crecimiento de once especies leñosas del matorral espinoso tamaulipeco en Linares, Nuevo León, México. Tesis de Maestría en Ciencias Forestales. Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. Linares, NL, México. 119 p. [ Links ]
García, D. and D. Martínez. 2012. Species richness matters for the quality of ecosystem services: a test using seed dispersal by frugivorous birds. Proceedings of the Royal Society B 279(1740):3106-3113. Doi: 10.1098/rspb.2012.0175. [ Links ]
García, E. 2004. Modificaciones al sistema de clasificación climática de Köppen para adaptarlo a las condiciones de la República Mexicana. Instituto de Geografía de la Universidad Nacional Autónoma de México. Coyoacán, México D. F., México. 98 p. [ Links ]
Gentry, A. H. 1982. Patterns of neotropical plant species diversity. In: Hecht, M. K., B. Wallace and G. T. Prance (Edits.). Evolutionary Biology. Springer. New York, NY, United States of America. pp. 1-84. [ Links ]
González‐Varo, J. P., C. S. Carvalho, J. M. Arroyo and P. Jordano. 2017. Unravelling seed dispersal through fragmented landscapes: frugivore species operate unevenly as mobile links. Molecular Ecology 26(16):4309-4321. Doi: 10.1111/mec.14181. [ Links ]
Gordo, O. and J. J. Sanz. 2009. Long‐term temporal changes of plant phenology in the Western Mediterranean. Global Change Biology 15(8):1930-1948. Doi: 10.1111/j.1365-2486.2009.01851.x. [ Links ]
Guevara, S., J. Laborde y G. Sánchez-Ríos. 2005. Los árboles que la selva dejó atrás. Interciencia 30(10):595-601. https://www.redalyc.org/pdf/339/33910903.pdf . (31 de julio de 2023). [ Links ]
Hadinezhad, M., R. Erfanzadeh and H. Ghelichnia. 2021. Soil seed bank characteristics in relation to different shrub species in semiarid regions. Land Degradation & Development 32(5):2025-2036. Doi: 10.1002/ldr.3856. [ Links ]
International Business Machines (IBM). 2022. IBM SPSS Statistics 25 Documentation. International Business Machines Corp. New York, NY, United States of America. https://www.ibm.com/support/pages/ibm-spss-statistics-25-documentation#es . (1 de agosto 2023). [ Links ]
Jurado, E. and J. Flores. 2005. Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16(5):559-564. Doi: 10.1111/j.1654-1103.2005.tb02396.x. [ Links ]
Jurado, E., E. Estrada and A. Moles̀. 2001a. Characterizing plant attributes with particular emphasis on seeds in Tamaulipan thornscrub in semi-arid Mexico. Journal of Arid Environments 48(3):309-321. Doi: 10.1006/jare.2000.0762. [ Links ]
Jurado, E., J. Navar, H. Villalón and M. Pando. 2001b. Germination associated with season and sunlight for Tamaulipan thornscrub plants in north-eastern Mexico. Journal of arid Environments 49(4):833-841. Doi: 10.1006/jare.2001.0817. [ Links ]
Jurado, E., J. Flores, A. G. Endress, M. Flores, E. Estrada and M. Pando. 2006. Seed removal rates under isolated trees and continuous vegetation in semiarid thornscrub. Restoration Ecology 14(2):204-209. Doi: 10.1111/j.1526-100X.2006.00122.x. [ Links ]
Levin, S. A. and H. C. Muller-Landau. 2000. The evolution of dispersal and seed size in plant communities. Evolutionary Ecology Research 2:409-435. https://repository.si.edu/bitstream/handle/10088/18533/stri_Levin_and_ML2000EER.pdf?sequence=1&isAllowed=y . (4 de mayo de 2023). [ Links ]
Ma, M., S. L. Collins, Z. Ratajczak and G. Du. 2021. Soil seed banks, alternative stable state theory, and ecosystem resilience. BioScience 71(7):697-707. Doi: 10.1093/biosci/biab011. [ Links ]
Mao, R., T. L. T. Nguyen, O. O. Osunkoya and S. W. Adkins. 2019. Spread pathways of the invasive weed Parthenium hysterophorus L.: The potential for water dispersal. Austral Ecology A Journal of ecology in the Southern Hemisphere 44(7):1111-1122. Doi: 10.1111/aec.12774. [ Links ]
Martínez-Adriano, C. A., E. Jurado, J. Flores, E. Estrada-Castillón and H. Gonzalez-Rodríguez. 2021. Effect of induced warming on seedling emergence of Tamaulipan thornscrub at northeastern Mexico. Flora 285:151965. Doi: 10.1016/j.flora.2021.151965. [ Links ]
Moles, A. T., D. D. Ackerly, C. O. Webb, J. C. Tweddle, J. B. Dickie and M. Westoby. 2005. A brief history of seed size. Science 307(5709):576-580. Doi: 10.1126/science.1104863. [ Links ]
Moles, A. T., D. S. Falster, M. R. Leishman and M. Westoby. 2004. Small‐seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. Journal of Ecology 92(3):384-396. Doi: 10.1111/j.0022-0477.2004.00880.x. [ Links ]
Molina-Guerra, V. M., M. Pando-Moreno, E. Alanís-Rodríguez, P. A. Canizales-Velázquez, H. González R. y J. Jiménez-Pérez. 2013. Composición y diversidad vegetal de dos sistemas de pastoreo en el matorral espinoso tamaulipeco del Noreste de México. Revista Mexicana de Ciencias Pecuarias 4(3):361-371. https://www.scielo.org.mx/pdf/rmcp/v4n3/v4n3a7.pdf . (2 de marzo de 2023). [ Links ]
Mora D., C. A., J. Jiménez P., E. Alanís R., E. A. Rubio C., J. I. Yerena Y. y M. A. González T. 2013. Efecto de la ganadería en la composición y diversidad arbórea y arbustiva del matorral espinoso tamaulipeco. Revista Mexicana de Ciencias Forestales 4(17):124-137. Doi: 10.29298/rmcf.v4i17.426. [ Links ]
Mori, S. A. and J. L. Brown. 1998. Epizoochorous dispersal by barbs, hooks, and spines in a lowland moist forest in central French Guiana. Brittonia 50:165-173. https://link.springer.com/article/10.2307/2807846 . (31 de julio de 2023). [ Links ]
Ogle, C. 2023. Cyclospermum leptophyllum. New Zealand Plant Conservation Network. https://www.nzpcn.org.nz/flora/species/cyclospermum-leptophyllum/. (23 de febrero de 2023). [ Links ]
Pando-Moreno, M., E. Jurado, D. Castillo, J. Flores and E. Estrada. 2010. Physical crust does not affect soil seed bank. Arid Land Research and Management 24(3):263-266. Doi: 10.1080/15324981003744966. [ Links ]
Pérez-Domínguez, R., E. Jurado, M. A. González-Tagle, J. Flores, O. A. Aguirre-Calderón y M. Pando-Moreno. 2013. Germinación de especies del matorral espinoso tamaulipeco en un gradiente de altitud. Revista Mexicana de Ciencias Forestales 4(17):156-163. Doi: 10.29298/rmcf.v4i17.428. [ Links ]
Pijl, L. 1969. Principles of dispersal in higher plants. Springer-Verlag. Heidelberg, BW, Germany. 214 p. https://link.springer.com/book/10.1007/978-3-662-00799-0 . (31 de julio de 2023). [ Links ]
Piudo, M. J. y R. Y. Cavero. 2005. Banco de semillas: comparación de metodologías de extracción, de densidad y de profundidad de muestreo. Publicaciones de Biología, Universidad de Navarra, Serie Botánica 16:71-85. https://dadun.unav.edu/bitstream/10171/8024/1/n16a5.pdf . (29 de abril de 2023). [ Links ]
Quevedo-Robledo, L., E. Pucheta and Y. Ribas-Fernández. 2010. Influences of interyear rainfall variability and microhabitat on the germinable seed bank of annual plants in a sandy Monte Desert. Journal of Arid Environments 74(2):167-172. Doi: 10.1016/j.jaridenv.2009.08.002. [ Links ]
Rodríguez‐Pérez, J., D. García and D. Martínez. 2014. Spatial networks of fleshy‐fruited trees drive the flow of avian seed dispersal through a landscape. Functional Ecology 28(4):990-998. Doi: 10.1111/1365-2435.12276. [ Links ]
Saatkamp, A., P. Poschlod and D. L. Venable. 2013. The functional role of soil seed banks in natural communities. In: Gallagher, R. S. (Edit.). Seeds: The ecology of regeneration in plant communities. CABI. Wallingford, OX, United Kingdom. pp. 263-295. [ Links ]
Sarmiento-Muñoz, T. I., E. Alanís-Rodríguez, J. M. Mata-Balderas y A. Mora-Olivo. 2019. Estructura y diversidad de la vegetación leñosa en un área de matorral espinoso tamaulipeco con actividad pecuaria en Nuevo León, México. CienciaUAT 14(1):31-44. Doi: 10.29059/cienciauat.v14i1.1001. [ Links ]
Shiferaw, W., S. Demissew and T. Bekele. 2018. Ecology of soil seed banks: Implications for conservation and restoration of natural vegetation: A review. International Journal of Biodiversity and Conservation 10(10):380-393. Doi: 10.5897/IJBC2018.1226. [ Links ]
Society for Ecological Restoration (SER) and Royal Botanic Gardens Kew (RBGK). 2023. Seed Information Database. https://ser-sid.org/. (23 de febrero de 2023). [ Links ]
Universidad de Sonora (Unison) y Universidad Estatal de Arizona (ASU). 2023. Red de Herbarios del Noroeste de México. https://herbanwmex.net/portal/index.php. (25 de febrero de 2023). [ Links ]
Valdes-Alameda, R., E. Jurado, J. Flores, M. Pando-Moreno, E. Estrada y D. E. Gurvich. 2021. Densidad de semillas y plántulas de Zanthoxylum fagara en México y Zanthoxylum coco en Argentina: influencia de plantas bajo las cuales ocurren y borde de la vegetación. Botanical Sciences 99(1):67-79. Doi: 10.17129/botsci.2636. [ Links ]
Valerio, C. E. e I. Moreira. 1986. Fenología de compuestas herbáceas (Compositae) en el Parque del Este, Costa Rica. Revista de Biología Tropical 34(1):161-163. https://revistas.ucr.ac.cr/index.php/rbt/article/view/24404/24532. (31 de julio de 2023). [ Links ]
Vargas-Mendoza, C. F., I. Ortegón-Campos, D. Marrufo-Zapata, C. M. Herrera and V. Parra-Tabla. 2015. Genetic diversity, outcrossing rate, and demographic history along a climatic gradient in the ruderal plant Ruellia nudiflora (Acanthaceae). Revista Mexicana de Biodiversidad 86(2):508-520. Doi: 10.1016/j.rmb.2015.04.034. [ Links ]
Waitman, B. A., S. B. Vander Wall and T. C. Esque. 2012. Seed dispersal and seed fate in Joshua tree (Yucca brevifolia). Journal of Arid Environments 81:1-8. Doi: 10.1016/j.jaridenv.2011.12.012. [ Links ]
Warnock, A. D., M. E. Westbrooke, S. K. Florentine and C. P. Hurst. 2007. Does Geijera parviflora Lindl. (Rutaceae) facilitate understorey species in semi-arid Australia? The Rangeland Journal 29(2):207-216. Doi: 10.1071/RJ07032. [ Links ]
Williams, J. T. 1963. Biological flora of the British Isles: Chenopodium album L. Journal of Ecology 51(3):711-725. https://www.jstor.org/stable/2257758?origin=crossref . (31 de julio de 2023). [ Links ]
Willson, M. F., B. L. Rice and M. Westoby. 1990. Seed dispersal spectra: a comparison of temperate plant communities. Journal of Vegetation Science 1(4):547-562. Doi: 10.2307/3235789. [ Links ]
Zar, J. H. 1999. Biostatistical analysis. Prentice Hall. Upper Saddle River, NJ, United States of America. 663 p. [ Links ]
Received: March 01, 2023; Accepted: May 10, 2023











 texto em
texto em