Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.12 no.66 México jul./ago. 2021 Epub 04-Oct-2021
https://doi.org/10.29298/rmcf.v12i66.819
Scientific article
Ectomycorrhizae morphotypes in a Pinus patula Schiede ex Schltdl. & Cham. stand chronosequence in eastern Mexico
1Universidad Veracruzana, Campus Xalapa. Facultad de Ciencias Agrícolas. México.
2Universidad Veracruzana Centro de Investigación en Micología Aplicada. México.
Forest activity for timber purposes in Mexico is based on the harvesting of various pine species, one of the mostly used is Pinus patula. Ectomycorrhizal (ECM) fungi increase seedling survival, and, therefore, their use ensures the success of forest plantations. Reforestation with native pine species increases the diversity of ectomycorrhizal fungi. The aim of this work was to compare species diversity associated with stand age and to determine the changes in the fungal community at the morphotype level. The study was carried out in a commercial plantation, with stands aged 2, 5, 7, 9, 14 to 25 and > 50 years. Mycorrhizal roots were collected at the beginning of the rainy season and grouped according to their morphology, and the diversity index, percentage of abundance and relative frequency were determined. Twenty-eight morphotypes were determined, including two of the Laccaria genus, two of Tomentella and one of Cortinarius. There is a positive correspondence between the number of morphotypes and the age of the pines. Mature stands (14-25 and >50 yr-old) were more diverse than young (2 to 9 yr-old) stands, with the exception of the 7 year-old stand, which showed similar diversity values to the mature stands. Probable morphotypes of Laccaria spp. were more abundant and frequent in the youngest stand (33 %). Throughout the chronosequence, unique and shared morphotypes were recorded. This work requires further research; however, it shows that stand age is an important factor in the composition of the ECM fungal communities and a key element in forest management plans.
Key words: Morphotypes; Pinus; plantation; reforestation; ectomycorrhizal symbiosis; fungal succession
La actividad forestal en México se sustenta en el aprovechamiento de diversas especies de pinos; una de las más utilizados es Pinus patula. Los hongos ectomicorrízicos (HECM) incrementan la supervivencia de las plántulas, por lo que su uso asegura el éxito de las plantaciones; además, la reforestación con taxa nativos de pino aumenta la diversidad de HECM. Los objetivos de este trabajo fueron comparar la diversidad de HECM asociados a la edad de los rodales y determinar los cambios en la comunidad fúngica a nivel de morfotipos. El estudio se realizó en una plantación con rodales de 2, 5, 7, 9, 14 a 25 y >50 años. Se recolectaron raíces micorrizadas al inicio de la temporada de lluvias, se agruparon de acuerdo con su morfología y se calculó el índice de diversidad, porcentaje de abundancia y frecuencia relativa. Se determinaron 28 morfotipos, tres a nivel de género: Laccaria (2), Tomentella (2) y Cortinarius (1). Se obtuvo una correspondencia positiva entre el número de morfotipos y la edad del arbolado. Los rodales maduros (14-25 y >50 años) fueron más diversos, con respecto a los jóvenes (2 a 9 años); a excepción del de 7 años, cuyos valores coincidieron con los rodales maduros. Los morfotipos probables de Laccaria spp. fueron más abundantes y frecuentes en el rodal más joven (33 %). A través de la cronosecuencia se registraron morfotipos únicos y compartidos. Se concluye que la edad del rodal es un factor importante en la composición de la comunidad HECM, elementos clave en los planes de manejo forestal.
Palabras clave: Morfotipos; Pinus; plantación; reforestación; simbiosis ectomicorrízica; sucesión fúngica
Introduction
Pinus patula Schiede ex Schltdl. & Cham. is one of the most commonly used subtropical species for reforestation in Mexico, with a high commercial value due to its fast growth and wood quality (Aparicio-Rentería, 2014). Forest activity in the country is based on the harvesting of some pine species in commercial forestry plantations, which represents 5% of timber production at the national level (López-López and Caballero et al., 2018). Due to this utilization, these plantations have short intervals for rotations, and these changes in the age classes (Spake et al., 2015) reduce the richness of ectomycorrhizal (ECM) fungi (Kranabetter et al., 2005).
Ectomycorrhizal symbiosis plays an important role in the acquisition of water and nutrients, mainly in species of the Pinaceae family, which facilitates the establishment and survival of seedlings (Guo et al., 2020). ECM fungal communities are made up of assemblages of species that change in space and time (Peay et al., 2010). Changes in the composition of ECM fungal communities are related to stand development; thus, stand age is a key factor in the dynamics of ECM fungi (Bonet et al., 2004).
Fungal taxa are classified into two succession patterns under a model of adaptive strategies. The first consists of early-stage species that thrive in areas with high disturbance and low stress intensity, such as Hebeloma (Fr.) P. Kumm, Lactarius Pers. e Inocybe (Fr.) Fr., which colonize the seedlings by means of spores that remain in the form of resistant propagules in the ground (Nara, 2008). The second includes late stage taxa, found in the roots closer to the trunk, associated with older and larger individuals (Nara et al., 2003a); belonging to such genera as Boletus, Russula and Cortinarius, which have the ability to colonize new hosts through extraradical mycelium; in addition, some Amanita species have been recorded in mature sites as cointroduced (Taylor and Bruns, 1999; Vlk et al., 2020).
These succession patterns have been evaluated as a function of both sporomes (Jones et al., 2003; Nara et al., 2003a; Fernández-Toirán et al., 2006; Dejene et al., 2017; Gómez-Hernández et al., 2019), as well as mycorrhizal root morphotypes (Nara et al., 2003b; Horton et al., 2005; Palfner et al., 2005; Ashkannejhad and Horton, 2006; Peay et al., 2010; Reverchon et al., 2010; Ma et al., 2012, Palacios et al., 2012). For example, an increase in morphotype diversity was observed in 30 to 60 year old stands in Picea A. Dietr. (Palfner et al., 2005).
Other authors report an increase in the diversity of ECM fungi at the morphotype level in Pseudotsuga menziesii (Mirb.) Franco in 40 and 400 year old stands (Horton et al., 2005) and in Pinus densiflora Siebold & Zucc., in 55 and 80 year-old trees (Ma et al., 2012) over a chronosequence. Guo et al. (2020) indicate that diversity of ECM fungi increased significantly with stand age, and the structure of the ECM fungal communities differs between age groups in 26, 33, and 43 year-old Pinus sylvestris var. mongolica Litv. stands, in all of which the Wilcoxina genus was the most dominant.
Species belonging to the Russulaceae and Thelephoraceae families are dominant in late successional stages of temperate ecosystems, where they play a critical role in nutrient cycling and are more adapted to the particular climatic conditions of the site (Erland y Taylor, 2002; Koizumi et al., 2018).
As a result of reforestation with Pinus spp. in regions outside their range, some species of ECM fungi have been introduced into areas where their native host is not present, or where the existing native ECM propagules are incompatible with the introduced host. According to the review by Vellinga et al. (2009) and Vlk et al. (2020), around 300 introduced ECM species have been identified worldwide; with at least 54 genera recorded in pine plantations, among them: Amanita, Astraeus, Boletus, Clavulina, Cortinarius, Gautieria, Gomphidius, Hebeloma, Inocybe, Laccaria, Lactarius, Pisolithus, Ramaria, Russula, Scleroderma, Suillus, Thelephora, and Tricholoma. Taxa belonging to Wicoxina, Suillus, Rhizopogon, Laccaria, Pisolithus, and Scleroderma have ecological adaptations (e.g., increased germination rate and spore longevity) that allow them to establish in early stages associated with hosts of the Pinaceaceae family, primarily.
Plantations with introduced hosts show a low ECM fungi richness (<50 taxa) in regard to the plantations established with native hosts (Ning et al., 2020). Within this context, Alem et al. (2020) document that, in a Pinus patula plantation in Ethiopia with different age classes (5, 11 and 36 years), 41 % of the operational taxonomic units (OTU) of the fungi identified were saprobes, 7 % were pathogens, and 2 % were ECM fungi.
In Mexico, although P. patula is a native species, fungal succession patterns in forest plantations have been little studied. Gómez-Hernández et al. (2019) identified a total of 63 taxa of EMF fungi and 43 saprobes in 1-, 11-, and 60-year-old stands, based solely on sporome sequencing, and Ramírez-Miguel et al. (2021) cited 14 ECM morphotypes in P. patula and Q. crassifolia roots; of these, Lactarius sp., Cenococcum geophilum Fr. and Tomentella radiosa (P. Karst.) Rick were the most frequent in both hosts.
Analyses based on both morphotype and sporome sequencing and morphological characterization of morphotypes and sporomes provide insight into the adaptive strategies of ECM fungi to establish in areas and hosts outside and within their distribution area. Although the morphological characterization of mycorrhizal roots has some methodological disadvantages, such as sample evaluation time and low identification to species level, in contrast to the advantages offered by molecular techniques on the latter aspect. This technique is used as a key strategy in nursery plant production, when controlled inoculations of ECM fungi are applied (Galindo-Flores et al., 2015), and also as an important complement in studies on ectomycorrhizal symbiosis.
The objective of this study was to determine the changes in the ECM fungal community at the ectomycorrhizal level in stands of different ages in an established plantation of P. patula. Furthermore, this study highlights the importance of forest plantations as refuges of great fungal diversity and the high potential of these fungi to be selected in future research as forest inoculants of P. patula, which will favor a greater success in the establishment of its plantations in sites within and outside its natural distribution area.
Materials and Methods
Sampling site
Sampling was carried out in a Pinus patula plantation in Coxmatla, Xico municipality, Veracruz State (19°25'53.29" N, 97°04'47.91" W); at 2 209 masl, with 25 h (Figure 1). The plantation was established in 2010 with seeds from the region and plants produced in local nurseries. These sites correspond to the stand that had reached the age of 9 years by the sampling date, in 2019. The 7-year-old site was planted in 2012, the 5-year-old, site in 2014, and the 2-year-old site, in 2017. The 14-25 and 50+ year old areas were established as permanent measurement and observation sites to collect data on wood increments and volumes. All stands are pure P. patula and have a density of 1 100 plants per hectare at 3 × 3 m.
The 5, 7 and 9 year old sites for 2019 had a 30 % thinning. Only dry, malformed trees, and some suppressed ones, have been extracted from older stands. The total area of the plantation is characterized by natural relicts of P. patula and P. pseudostrobus Lindl. with a history of land use for cattle ranching. The soil exhibits compaction and degradation issues; however, during the last decade the plantation has been dedicated for conservation and research.
Sampling of roots
Six age categories were established: 2, 5, 7, 9, 14-25 and >50 years, and roots were collected from 10 randomly selected trees in each stand (Table 1); sampling was carried out during July 2018. For each individual, three replications were obtained, in the shape of a triangle, at a distance of 25 cm from the trunk (Guo et al., 2020 modified); organic matter was removed and a metal nucleator (AMS) with a 5 cm diameter and a depth of 20 cm was used (Figure 2).
Table 1 Description of the sampled stands.
| Stand | Age of the stand (years) |
Altitude (masl) |
Cordinates |
|---|---|---|---|
| S1 | 2 | 2 022 | 19°25'53.64120" N 97°04'47.77320" O |
| S2 | 5 | 2 008 | 19°25'52.32720" N 97°04'48.11880" O |
| S3 | 7 | 2 005 | 19°25'55.62480" N 97°04'42.85920" O |
| S4 | 9 | 2 018 | 19°25'50.71080" N 97°04'48.01800" O |
| S5 | 14-25 | 2 028 | 19°25'58.03680" N 97°04'37.15680" O |
| S6 | >50 | 2 014 | 19°25'53.90400" N 97°04'41.40120" O |

Figure 2 Distribution of stands within the Pinus patula Schiede ex Schltdl. & Cham. plantation (a); sampling (b).
The mycorrhizal tips were extracted from each sample. The largest roots were separated, and the remaining roots were washed and sieved with a 1 mm and a 2 mm sieve. These were dissected under a Leica EZ4 stereoscopic microscope, and roots with turgid mycorrhizae and with mantle were separated from the dead or non-mycorrhizal ones. They were carefully cleaned with distilled water. The morphological characters of mycorrhizae were described macroscopically in order to divide them into morphotypes according to their morphological characters, using the standard methodology of Agerer (1991): type of branching, color, tip branching, mantle color, rhizomorphs, and emanant hyphae. Each morphotype was photographed with a Motorola G8 camera with a 40X magnification.
Statistical analyses
The Shannon-Wiener (H') and Simpson (D') richness and diversity indices were estimated. These variables were compared among the different ages of the stands using a generalized linear model, LSD Fisher comparison test with a significance level of 95 %, after checking the assumptions of normality and heterogeneity of variance in the data. The Statistica 9.0 (StatSoft, Inc.), EstimateS 9.1.0, PAST softwares were used (Hammer et al., 2001).
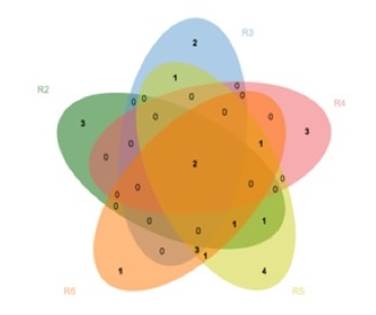
Relative abundance was calculated by dividing the number of mycorrhizal roots per morphotype by the total number of mycorrhizal roots for each stand. Relative frequency was estimated as the frequency of each morphotype divided by the sum of the frequencies of all morphotypes present per stand. The influence of stand age on the distribution of morphotypes according to their presence and abundance was determined by means of a detrended correspondence analysis (DCA). A Venn diagram was constructed to compare the shared and exclusive morphotypes per stand.
Results
A total of 1 849 mycorrhizal tips were assessed; from a total of 30 samples per stand studied, which morphologically corresponded to 28 morphotypes. Five morphotypes were tentatively identified at the genus level: Cortinarius sp., Laccaria spp., and Tomentella spp. (Figure 3); the remaining ones could not be identified due to the absence of diagnostic characteristics.

(a) Laccaria spp.; (b, c) Unidentified morphotype (M6, M7); (d, e) Tomentella spp., (f) Cortinarius sp. (scale bar = 1 mm).
Figure 3 Morphology of the most frequent ectomycorrhizae and their probable identification in a chronosequence of Pinus patula Schiede ex Schltdl. & Cham.
The stand with the highest richness of morphotypes was S5 with 14, which corresponded to 50 % of the total recorded; followed by S6, with 9 morphotypes (32.1 %); S3 with 8 morphotypes (28.5 %); S2 with 7 morphotypes (25 %); S4 with 6 morphotypes (21.4 %), and S1 with 5 morphotypes (17.8 %) (Figure 4, Table 2).

Figure 4 Relative abundance (a) and relative frequency (b) of morphotypes of EcM fungi in six stands of Pinus patula Schiede ex Schltdl. & Cham. of different ages
Table 2 EMF community α-diversity indices (based on morphotypes) in Pinus patula Schiede ex Schltdl. & Cham. stands of different ages.
| Stand | Number of morphotypes |
Total number of mycorrhizal tips analyzed |
Morphotypes |
Simpson (D´) |
Shannon Wiener (H’) |
|---|---|---|---|---|---|
| S1 | 5 | 94c | M1, Laccaria spp., M3, M4, M5 | 0.76±0.04 | 1.53±0.07d |
| S2 | 7 | 360b | M6, M7, M8, M9, M10, M11, Laccaria spp. |
0.81±0.06 | 1.79±0.02c |
| S3 | 8 | 326bc | M6, M8, Tomentella spp., M14, M16, M17, M18 |
0.86±0.08 | 2.02±0.05b |
| S4 | 6 | 668a | M6, M8, Cortinarius sp., M20, M21, M22 |
0.81±0.05 | 1.74±0.02c |
| S5 | 14 | 225bc | M6, M7, M8, Laccaria spp., Tomentella spp., M17, M18, Cortinarius sp., M23, M24, M25, M26, M27 |
0.78±0.04 | 2.07±0.06a |
| S6 | 9 | 176bc | M6, M8, Laccaria spp., Tomentella spp., M18, Cortinarius sp., M23, M28 |
0.86±0.06 | 2.08±0.05a |
The α-diversity values represent the averages per 30 samples per stand (three replicates per tree per stand). Letters represent significant differences (P < 0.05).
Significant differences were observed in the abundance of ECM fungi with respect to stand age (P = 0.002). The highest total abundance of mycorrhizal apices was found in S4, with 36 % (668); followed by S2, with 19.4 % (360); S3, with 17.6 % (326); S5, with 12.16 % (225); S6, with 9.5 % (176), and S1, with 5.08 % (94) (Figure 4).
The probable morphotype of Laccaria spp. exhibited a relative abundance (Ai) of 33.9 % in the 2-year old stand (S1) and a relative frequency (Fi) of 38.8 %. In S2, morphotype M10 showed the highest values for Ai (29.44 %) and Fi (13.04 %). Morphotypes M6 (Ai=19.3 %) and M14 (Ai=17.1 %) had the highest values in S3; however, M14 exhibited a relative frequency of 3.8 %, the lowest for this stand. In S4, M20 had a high Ai value (27.5 %), with a Fi of 19.23 %. Stands S5 and S6 shared a large number of morphotypes, with a dominance of morphotype M26 in S5, whose Ai was 41.9 % and Fi of 12.28 %. In S6, the relative abundance and frequency for each morphotype was similar. Morphotypes M6 and M8 were identified in all stands, except in S1 (2 years), where they contributed 23.4 % of the total abundance (Figure 4).
The α-diversity indices (Table 2) indicated an increase in diversity in relation to stand age; the older stands S5 and S6 (H' = 2.07±0.06, H' = 2.08±0.05) exhibited higher values compared to the younger ones, with the exception of S3, which had a similar H' index to the one estimated for stands S5 and S6.
The detrended correspondence analysis (DCA) shows the distribution of all morphotypes of ECM fungi in the different ages of the stands, and explains 78 % of the variability of the data in axes 2 (64 %) and 3 (14 %) (Figure 5). The graph shows the distribution of the morphotypes and divides them into four groups: G I consists of the morphotypes shared in S3, S5 and S6, among which many intermingled units are observed, suggesting a high similarity between their EMF morphotype assemblages. The second group (G II) is composed of M1, Laccaria spp., M3, M4 and M5 in the 2-year-old stand, which were not found in the other stands. A third group (G III), with M7, M9, M10 and M11, present only in the 5-year old stand; and a fourth group (G IV) of morphotypes distributed in the 14-25 year-old stand (S5).
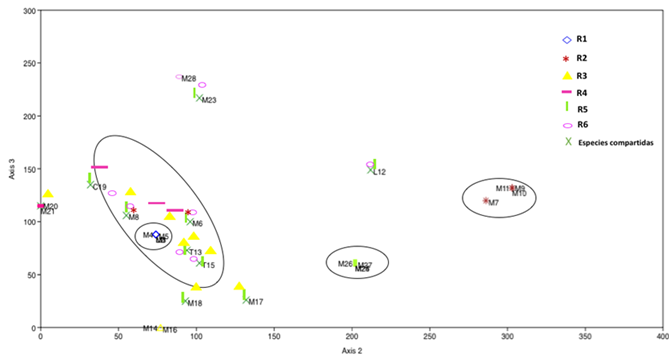
Figure 5 Detrended correspondence analysis (DCA) for EMF morphotypes present in six stands of different ages in a Pinus patula Schiede ex Schltdl. & Cham. plantation.
Throughout the chronosequence, unique and shared morphotypes were registered. Morphotypes M6 and M8 were observed in all stands, with the exception of S1, which had no morphotypes shared with the rest of the stands. The largest number of single morphotypes (4) was obtained in S5, while S6 included only one single morphotype (M28). Older stands (S5 and S6) shared a larger number of morphotypes (Figure 6).
Discussion
The α-diversity indexes showed differences between stand ages. The diversity at the morphotype level was expected to increase with stand age; although there was indeed an increasing trend in the 14-25 and >50 year-old stands, the results showed similar diversity values in the 7 year-old stand. This can be explained by the fact that the 7-year-old stand has had a 30 % thinning, and, thus, more light was allowed to enter the understory plants, which contribute to improve the availability of water and nutrients in the soil (Dang et al., 2018).
The 2-year-old stand has not yet received the first thinning and has a higher density of trees and, therefore, less light input, which may have resulted in the low number of morphotypes. Gómez Hernández et al. (2019) observed a similar pattern in intermediate stages of development in 11 year-old plantations of P. patula and Quercus rugosa Neé, where the diversity of ECM fungal species was similar to that of mature (60-year-old) stands with a mixed tree composition (P. patula, P. douglasiana Martínez, P. teocote Schiede ex Schltdl. & Cham., Q. rugosa, and Q. laurina Bonpl.), while it decreased in young stands (1 year) consisting exclusively of P. patula. The authors point out that canopy development and tree density are important factors in sporome production and in the increase in the diversity of ECM fungi.
Although the present study is exploratory, its results show that succession processes directly influence ECM fungi and that diversity is modulated by the time of plantation establishment. Older stands tend to provide better habitats for ECM fungal species than younger, heterogeneous stands. Unlike cited by Dejene et al. (2017) in sites where the Pinus genus is an introduced species, few species of ECM fungi and low diversity associated with P. patula are registered, due to the small amount of compatible propagules in the soil. These authors note that they found no ECM fungal species in 5-year-old stands, but cite an increase in sporomes of ECM fungi in 11 to 36-year-old stands.
Alem et al. (2020) suggest that thinning and previous land use are the main factors modulating edaphic ECM fungal communities in P. patula. These authors suggest that 5 and 36 year-old stands are more diverse in terms of ECM fungal species than 11 year-old intermediate stands, due to the poor development of the canopy and to the possibility that the site was previously used for agricultural purposes. For this reason, it has a high number of propagules associated with young stages, from the spore bank of adjacent plantations. The same has been observed in other conifers planted in areas outside their natural distribution range, such as Pinus radiata D. Don. This species has been studied in 20-year-old stands, which included a total of 11 morphotypes and exhibited a low diversity of ECM fungi, compared to 5 and 10 year-old stands, the latter of which has a lower canopy density (Palacios et al., 2012).
The highest values for diversity of ECM fungal species were found in the 14-25 years old and >50 years old stands, which reflects the difference between the forestry interventions applied. Parladé et al. (2017) and Tomao et al. (2017) mention that the intensity of partial thinning is a factor that favors the appearance of ECM fungi sporomes but does not necessarily affect species diversity at the root level (Castaño et al., 2018). Although this parameter was not directly evaluated in the study, it should be considered under different soil and climatic conditions in order to model trends regarding its effect on other functional groups of fungal diversity.
Figure 5 shows that, in the 7, 14-25, and >50 year old stands, most of the morphotypes are shared, which implies that the age of the stand does not necessarily determine either the diversity or the composition of the ECM fungal community. Castaño et al. (2019) point out that the diversity and abundance of ECM fungi species increase with tree age, but are directly affected by the availability of N and P, as well as by the increase in the C/N ratio.
In the present work, a high number of morphotypes of ECM fungi associated with a plantation of P. patula were registered through the use of morphological characterization of ectomycorrhizae. This method has limitations due to the difficulty in differentiating between species, which generates the underestimation or overestimation of the wealth of species associated with a host. Undoubtedly, molecular methods are useful in the identification of these ECM fungal communities, as they allow to clarify the identity of fungal taxa. However, morphological methods are a first approach to the differentiation of replacement and dominance patterns of species associated with hosts, about which ―it should be noted― little or no information is available. Such is the case of P. patula, a taxon with a high potential for forest exploitation within and outside its natural distribution range.
Some morphotypes have been described in Mexico, at early stages of P. patula: Boletus edullis Bull., Laccaria bicolor (Maire) P.D. Orton, L. proxima (Boud.) Pat., Hebeloma alpinum (J. Favre) Bruchet, H. leucosarx P.D. Orton, H. mesophaeum (Pers.) Quél, and Suillus pseudobrevipes A.H. Sm. & Thiers (Carrera-Nieva and López-Ríos, 2004; Carrasco-Hernández et al., 2010; Jiménez, 2011), mainly in experiments with controlled inoculation. However, this is one of the first studies to explore mycorrhizal roots at the morphotype level, within a chronosequence in stands of the same host. Yet, further research, at the molecular level, is still required (Ramírez-Miguel et al., 2021).
Ectomycorrhizal fungi play a structural role in determining the conditions for the establishment and development of different forest species (Pérez-Moreno et al., 2020). Therefore, it is essential to assess fungal diversity in plantation forests from the perspective of understanding such forestry practices as partial logging, low intensity thinning (30-50 %), mixing of native plant varieties, and the development of stands that promote interconnectivity within the forest matrix and ensure the establishment of pioneer ECM fungal species.
Conclusions
The diversity of morphotypes of ECM fungi is related to stand age, although it is not the only factor. The highest diversity values were found in 7, 14-25 and >50 year old stands. The composition of ECM fungal communities is clustered into single morphotypes in the youngest stand and similar ectomycorrhizas among the 7, 9, 14-25 and >50 year old sites. The most abundant and frequent morphotypes of ECM fungi in roots of P. patula were determined, describing Laccaria as one of the most abundant in the youngest sites. This work highlights the importance of forest plantations as refuges of fungal diversity.
Acknowledgements
The authors would like to thank the Laboratory of Beneficial Organisms of Universidad Veracruzana for providing the means to process the samples, as well as J. Dorantes, for his valuable support in fieldwork and the facilities provided. All authors thank the reviewers and the editor for their timely comments, which contributed to the improvement of the manuscript.
REFERENCES
Agerer, R. 1991. Characterization of ectomycorrhiza. Methods in microbiology 23: 25-73. Doi: 10.1016/S0580-9517(08)70172-7. [ Links ]
Alem, D., T. Dejene, J. A. Oria-de-Rueda, J. Geml and P. Martín-Pinto. 2020. Soil Fungal Communities under Pinus patula Schiede ex Schltdl. & Cham. Plantation Forests of Different Ages in Ethiopia. Forests 11(10): 1109. Doi: 10.3390/f11101109. [ Links ]
Aparicio-Rentería, A., S. F. Juárez-Cerrillo y L. R. Sánchez-Velásquez. 2014. Propagación por enraizamiento de estacas y conservación de árboles plus extintos de Pinus patula procedentes del norte de Veracruz, México. Madera y Bosques 20 (1): 85-96. Doi: 10.21829/myb.2014.201178. [ Links ]
Ashkannejhad, S. and T. R. Horton. 2006. Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytologist 169 (2): 345-354. Doi: 10.1111/j.1469-8137.2005.01593.x. [ Links ]
Bonet, J. A., C. R. Fischer and C. Colinas. 2004. The relationship between forest age and aspect on the production of sporocarps of ectomycorrhizal fungi in Pinus sylvestris forests of the central Pyreness. Forest Ecology and Management 203: 157-175. Doi: 10.1016/j.foreco.2004.07.063. [ Links ]
Carrasco-Hernández, V., J. Pérez-Moreno, V. Espinosa-Hernández, J. J. Almaraz-Suárez, R. Quintero-Lizaol y M. Torres-Aquino. 2010. Caracterización de micorrizas establecidas entre dos hongos comestibles silvestres y pinos nativos de México. Revista Mexicana de Ciencias Agrícolas 1(4): 567-577. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-09342010000400009&lng=es&tlng=es . (20 de junio de 2020). [ Links ]
Carrera-Nieva, A. y G. F. López-Ríos. 2004. Manejo y evaluación de ectomicorrizas en especies forestales. Revista Chapingo. Serie Ciencias Forestales y del Ambiente 10 (2): 93-98. https://www.redalyc.org/articulo.oa?id=629/62910204 (20 de junio de 2020). [ Links ]
Castaño, C., J. G. Alday, B. D. Lindahl, J. Martínez de Aragón, S. de-Miguel, C. Colinas, J. Parladé, J. Pera and J. A. Bonet. 2018. Lack of thinning effects over inter-annual changes in soil fungal community and diversity in a Mediterranean pine forest. Forest Ecology and Management 424:420-427. Doi:10.1016/j.foreco.2018.05.004. [ Links ]
Castaño, C. , T. Dejene , O. Mediavilla, J. Geml, J. A. Oria-de-Rueda and P. Martín-Pinto. 2019. Changes in fungal diversity and composition along a chronosequence of Eucalyptus grandis plantations in Ethiopia. Fungal ecology 39: 328-335. Doi: 10.1016/j.funeco.2019.02.003. [ Links ]
Dang, P., Y. Gao, J. Liu, S. Yu and Z. Zhao. 2018. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Science of the Total Environment 630: 171-180. Doi: 10.1016/j.scitotenv.2018.02.197. [ Links ]
Dejene, T., J. A. Oria-de-Rueda and P. Martín-Pinto. 2017. Fungal diversity and succession following stand development in Pinus patula Schiede ex Schltdl. & Cham. plantations in Ethiopia. Forest Ecology and Management 395: 9-18. Doi: 10.1016/j.foreco.2017.03.032. [ Links ]
Erland, S. and A. F. Taylor. 2002. Diversity of ectomycorrhizal fungal communities in relation to the abiotic environment. In: van der Heijden, M. G. A. and I. R. Sanders (eds.). Mycorrhizal ecology. Springer. Heidelberg, Berlin. pp. 163-200. Doi: 10.1007/978-3-540-38364-2. [ Links ]
Fernández-Toirán, L. M., T. Ágreda and J. M. Olano. 2006. Stand age and sampling year effect on the fungal fruit body community in Pinus pinaster forests in central Spain. Botany 84 (8): 1249-1258. Doi: 10.1139/b06-087. [ Links ]
Galindo-Flores, G., C. Castillo-Guevera, A. Campos-López y C. Lara. 2015. Caracterización de las ectomicorrizas formadas por Laccaria trichodermophora y Suillus tomentosus en Pinus montezumae. Botanical Sciences 93 (4): 855-86. Doi: 10.17129/botsci.200. [ Links ]
Gómez-Hernández, M., K. G. Ramírez-Antonio and E. Gándara. 2019. Ectomycorrhizal and wood-decay macromycete communities along development stages of managed Pinus patula stands in Southwest Mexico. Fungal ecology 39:109-116. Doi: 10.1016/j.funeco.2018.12.007. [ Links ]
Guo, M. S., G. D. Ding, G. L. Gao, Y. Zhang, H. Y. Cao and Y. Re. 2020. Community composition of ectomycorrhizal fungi associated with Pinus sylvestris var. mongolica plantations of various ages in the Horqin Sandy Land. Ecological Indicators 110: 105860. Doi: 10.1002/ece3.6119. [ Links ]
Hammer, Ø., D. A. Harper and P. D. Ryan. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia electronica 4 (1): 9 Palaeontologia electronica 4 (1): 9 http://palaeoelectronica.org/2001_1/past/issue1_01.htm (11 de febrero de 2020). [ Links ]
Horton, T. R., R. Molina y K. Hood. 2005. Douglas-fir ectomycorrhizae in 40-and 400-year-old stands: mycobiont availability to late successional western hemlock. Mycorrhiza 15 (6):393-403. Doi: 10.1007/s00572-004-0339-9. [ Links ]
Jiménez R., M. 2011. Estudio etnomicológico y biotecnológico de hongos silvestres comestibles ectomicorrízicos en Pinus pseudostrobus y evaluación de bacterias promotoras de crecimiento vegetal. Tesis de Maestría. Colegio de Posgraduados. Montecillo, Edo. de Méx., México. 120 p. [ Links ]
Jones, M. D., D. M. Durall and J. W. Cairney. 2003. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytologist 157 (3): 399-422. Doi: 10.1046/j.1469-8137.2003.00698.x [ Links ]
Koizumi, T., M. Hattori and K. Nara. 2018. Ectomycorrhizal fungal communities in alpine relict forests of Pinus pumila on Mt. Norikura, Japan. Mycorrhiza 28(2): 129-145. Doi: 10.1007/s00572-017-0817-5. [ Links ]
Kranabetter, J. M., J. Friesen, S. Gamiet and P. Kroeger. 2005. Ectomycorrhizal mushroom distribution by stand age in western hemlock-lodgepole pine forests of northwestern British Columbia. Canadian Journal of Forest Research 35 (7): 1527-1539. DOI: 10.1139/x05-095. [ Links ]
López L., M. Á. y M. Caballero D. 2018. Análisis financiero de una plantación de Pinus patula Schiede ex Schltdl. et Cham. de pequeña escala. Revista Mexicana de Ciencias Forestales 9(46):186-208. DOI: 10.29298/rmcf.v9i46.116. [ Links ]
Ma, D., Z. Shuying, W. Luhe and Z. Dongyou. 2012. Ectomycorrhiza community structure in chronosequences of Pinus densiflora in eastern China. African Journal of Microbiology Research 6 (32): 6204-6209. Doi: 10.5897/AJMR12.902. [ Links ]
Nara, K., H. Nakaya and T. Hogetsu. 2003a. Ectomycorrhizal sporocarp succession and production during early primary succession on Mount Fuji. New Phytologist 158: 193e206. Doi: 10.1046/j.1469-8137.2003.00724.x. [ Links ]
Nara, K. , H. Nakaya, B. Y. Wu, Z. H. Zhou and T. Hogetsu. 2003b. Underground primary succession of ectomycorrhizal fungi in a volcanic desert on Mount Fuji. New Phytologist 159: 743e756. Doi: 10.1046/j.1469-8137.2003.00844.x. [ Links ]
Nara, K. 2008. Community developmental patterns and ecological functions of ectomycorrhizal fungi: implications from primary succession. In: Varma, A. (ed.). Mycorrhiza. Springer. Heidelberg, Berlin. pp. 581-599. Doi: 10.1007/978-3-540-78826-3_28. [ Links ]
Ning, C., W. Xiang, G. M. Mueller, L. Egerton-Warburton, W. Yan and S. Liu. 2020. Differences in ectomycorrhizal community assembly between native and exotic pines are reflected in their enzymatic functional capacities. Plant and Soil, 446(1): 179-193. Doi: 10.1007/s11104-019-04355-9. [ Links ]
Palacios, Y., G. Palfner y C. Hernández. 2012. Comunidad ectomicorrícica en una cronosecuencia de Pinus radiata (Pinophyta: Pinaceae) de la zona de transición climática mediterráneo-templada de Chile central. Revista Chilena de Historia Natural 85 (1): 61-71. Doi: 10.4067/S0716-078X2012000100005. [ Links ]
Palfner, G., M. A. Casanova-Katny and D. J. Read. 2005. The mycorrhizal community in a forest chronosequence of Sitka spruce Picea sitchensis (Bong.) Carr. in Northern England. Mycorrhiza 15 (8): 571-579. Doi: 10.1007/s00572-005-0364-3. [ Links ]
Parladé, J., F. Martínez-Peña and J. Pera . 2017. Effects of forest management and climatic variables on the mycelium dynamics and sporocarp production of the ectomycorrhizal fungus Boletus edulis. Forest Ecology and Management 390: 73-79. Doi: 10.1016/j.foreco.2017.01.025. [ Links ]
Peay, K. G., P. G. Kennedy and T. D. Bruns. 2010. Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation. Fungal ecology 4 (3): 233-240. Doi: 10.1016/j.funeco.2010.09.010. [ Links ]
Pérez-Moreno, J., M. Martínez-Reyes and F. Hernández-Santiago.2020. Biotechnology of inoculation of trees of forest importance with edible ectomycorrhizal fungi Agroproductividad 13(5): 91-93. Doi 10.32854/agrop.vi.1540. [ Links ]
Ramírez-Miguel, A. A., A. F. Hernández-Díaz, C., Valenzuela-Encinas, R. Garibay-Orijel y C. Truong. 2021. Hongos ectomicorrízicos asociados a plantas jóvenes de Pinus patula y Quercus crassifolia en plantaciones del sistema matarrasa de la Sierra Juárez de Oaxaca, México. Scientia Fungorum 1-13. Doi: 10.33885/sf.2021.51.1289. [ Links ]
Reverchon, F., P. Ortega-Larrocea, J. Pérez-Moreno , V. Peña-Ramírez and C. Siebe. 2010. Changes in community structure of ectomycorrhizal fungi associated with Pinus montezumae across a volcanic soil chronosequence at Sierra Chichinautzin, Mexico. Canadian Journal of Forest Research 40 (6): 1165-1174. Doi: 10.1139/X10-062. [ Links ]
Spake, R., T. H. Ezard, P. A. Martin, A. C. Newton and C.P. Doncaster. 2015. A meta‐analysis of functional group responses to forest recovery outside of the tropics. Conservation Biology 29 (6): 1695-1703. Doi: 10.1111/cobi.12548. [ Links ]
Taylor, D. L. and T. D. Bruns. 1999. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Molecular Ecology 8(11): 1837-1850. Doi: 10.1046/j.1365-294x.1999.00773.x. [ Links ]
Tomao, A., J. A. Bonet , J. M. de Aragón and S. de-Miguel. 2017. Is silviculture able to enhance wild forest mushroom resources? Current knowledge and future perspectives. Forest Ecology and Management 402: 102-114. Doi: 10.1016/j.foreco.2019.117678. [ Links ]
Vellinga, E. C., B. E. Wolfe and A. Pringle. 2009. Global patterns of ectomycorrhizal introductions. New Phytologist 181(4): 960-973. Doi: 10.1111/j.1469-8137.2008.02728.x. [ Links ]
Vlk, L., L. Tedersoo, T. Antl, T. Větrovský, K. Abarenkov, J. Pergl, J. Albrechtová, M. Vosátka, P. Baldrian, P. Pysek and P. Kohout. 2020. Early successional ectomycorrhizal fungi are more likely to naturalize outside their native range than other ectomycorrhizal fungi. New Phytologist . Doi: 10.1111/nph.16557. [ Links ]
Received: July 29, 2020; Accepted: March 03, 2021











 texto en
texto en