Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias forestales
versão impressa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.12 no.63 México Jan./Fev. 2021 Epub 26-Mar-2021
https://doi.org/10.29298/rmcf.v12i63.762
Scientific article
Structure and diversity of a Helietta parvifolia (A. Gray ex Hemsl.) Benth.
1Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León. México.
3División de Desarrollo Sustentable, Universidad de Quintana Roo. México.
4Instituto de Ecología Aplicada, Universidad Autónoma de Tamaulipas. México.
Helietta parvifolia is a species of economic importance in Northern Mexico, being a potential source of wood and firewood for several uses. The objective of the study was to evaluate the structure and diversity of a mature H. parvifolia scrub in Northwest Mexico. Vegetation diversity was determined by establishing 20 sampling sites of 10 × 10 m each (2000 m² in total), where the normal and crown diameter of all trees and shrubs with a diameter ≥ 3 cm were recorded. The Margalef, Shannon and Pretzsch indexes were calculated for richness, species diversity and vertical structure, respectively. Eleven tree and shrub species were found, distributed in nine genera and six families. The Fabaceae family had the highest number of taxa (five), while the remaining families had only one species. Three of them concentrated 82.59 % of the importance value index values (IVI): Helietta parvifolia, Cordia boissieri and Havardia pallens, with 53 %, 15.44 % and 14.15%, respectively. The abundance of individuals of small diameter classes suggests the existence of an active regeneration state.
Keywords Species diversity; Helietta parvifolia (A. Gray ex Hemsl.) Benth.; submontane scrub; species richness; xerophilous vegetation
Helietta parvifolia es una especie de importancia económica en el norte de México, ya que es una fuente potencial de madera y leña para usos diversos. El objetivo del presente estudio fue evaluar la estructura y diversidad de un matorral maduro de H. parvifolia en el noroeste de México. Se determinó la diversidad de la vegetación mediante el establecimiento de 20 sitios de muestreo de 10 × 10 m cada uno (2 000 m² en total), en donde se registraron las especies, el diámetro normal y de copa de todos los árboles y arbustos con un diámetro ≥ 3 cm. Se calcularon los índices de Margalef, Shannon y Pretzsch para determinar la riqueza, diversidad de especies y estructura vertical, respectivamente. Se registraron 11 especies arbóreas y arbustivas, distribuidas en nueve géneros y seis familias. Fabaceae fue la que registró un mayor número de taxones (cinco); mientras que las familias restantes presentaron solamente un taxón. Tres especies concentraron 82.59 % de los valores de índice de valor de importancia (IVI): Helietta parvifolia, Cordia boissieri y Havardia pallens, con 53 %, 15.44 % y 14.15 %, respectivamente. La mayor abundancia de individuos de clases diamétricas pequeñas sugiere la existencia de un estado de regeneración activo.
Palabras clave Diversidad de especies; Helietta parvifolia (A. Gray ex Hemsl.) Benth.; matorral submontano; riqueza de especies; vegetación xerófila
Introduction
The xerophilous scrub constitutes one of the most important vegetal communities in Mexico. It occupies around 40 % of the national territory, and has a floristic richness estimated in about 6 000 species, including a high proportion of endemic species (Rzedowski, 1991, Semarnat, 2006). This plant community is a source of ecological, genetic, social, economic, educational, cultural, recreational and aesthetic wealth (Hooper et al., 2005; Santos and Tellería, 2006). Despite its importance, xerophytic scrub has changed in its structure and composition over time, so it has a loss of biological diversity that leads to a deficit in its functioning (Hooper et al., 2005). The above has its origin in multiple human activities, which have caused constant alterations in their coverage and changes in land use.
Within the xerophytic scrubland formations, there is the submontane scrubland, which is characterized by the development of hills and mountainsides below 2 000 masl (Rzedowski, 1978; González-Medrano, 2012). It thrives in shallow sedimentary or igneous soils and in semi-arid climates with precipitation values between 450 and 900 mm (Rojas-Mendoza, 1965). The submontane scrub is located along the Sierra Madre Oriental in the states of Hidalgo, Querétaro, San Luis Potosí, Tamaulipas, Coahuila and Nuevo León (Alanís-Rodríguez et al., 2015). In this last entity, 228 species of vascular plants have been documented, and it is in the central and southern areas where there is great plant richness (Estrada-Castillón et al., 2012).
Although the submontane scrub of northeastern Mexico is generally considered to be in a good state of conservation, it is evident that anthropogenic pressure is increasing for these types of communities, especially in areas close to urban areas (Alanís-Rodríguez et al., 2015; Mora-Olivo et al., 2016).
The conservation and sustainable management of plant communities requires knowledge of the functional and structural characteristics of the target vegetation (Montagnini and Jordan, 2005). For this reason, several studies assess the structure and composition of the submontane scrub in the northeast of the country, among them those by Foroughbakhch et al. (2003), Canizales-Velázquez et al. (2009), Estrada-Castillón et al. (2012), Alanís-Rodríguez et al. (2015) and Uvalle-Sauceda et al. (2015) for Nuevo León; in addition to that of Mora-Olivo et al. (2016) for Tamaulipas. However, so far these works are considered insufficient to attain a more accurate assessment of these scrublands, especially when they include in their composition species of high socioeconomic value, such as Helietta parvifolia (A. Gray) Benth. known as barreta.
Helietta parvifolia is one of the most important timber taxa in the submontane scrub of northeastern Mexico; its shaft is used by local people for the construction of fences due to its high quality and wood density. Derived from the above, research has been made to evaluate its timber production (Maginot et al., 2014), wood durability (Carrillo et al., 2013) and biomass (Návar et al., 2001), as well as its growth in forest plantations (Foroughbakhch, 1992). Therefore, the objective of this research was to analyze the structure and particularly the plant diversity of a mature submontane scrub where Helietta parvifolia is dominant in northwestern Mexico.
Materials and Methods
Study area
The study was carried out in the northwest of Linares municipality, Nuevo León, in a preserved area of the submontane scrub where Helietta parvifolia grows (Figure 1). The zone is located between 24°42'01.97'' N and 99°38'03.02'' W, at an altitude of 470 to 490 m, with a weighted average of 480 masl. According to data from INEGI (2009), the average annual temperature in the area fluctuates between 16 °C and 24 °C; the climate is a semi-warm - sub-humid with summer rains, of average humidity, and an average annual rainfall of 500 to 1 100 mm, with an average of 800 mm per year (INEGI, 2009). Based upon the information gathered, the vegetation in the study area is mature, without disturbances or previous clearing as a result of the forestry activities that dominate the region; the only use given to the plant community was the collection of edible fruits and woody material from the ground.
Field evaluation
In February 2019, 20 sampling sites of 100 m² (10 m × 10 m) were established, forming a total assessment area of 2 000 m². All scrub and tree components (normal diameter ≥ 3 cm) of each site were considered. The mensuration variables taken at the field were total height (h), which was measured with a Hastings® E-15-1 telescopic rod; normal diameter (d 1.30 ), with a 1270 mm Haglöf Mantax BlueTM caliper; and crown diameters (k) in N-S and E-W directions with a 10 m TruperTM tape. The species to which each individual belonged was recorded. Specialists from the Graduate School of Forest Sciences of the Universidad Autónoma de Nuevo León identified all species of trees and shrubs with a normal diameter ≥ 3 cm (Molina-Guerra et al., 2019).
Data analysis
The abundance of each taxon was determined according to the number of trees present, its coverage from the crown area, and its frequency based on its presence in each of the sampling frames. The variables in their relative form were used to obtain a taxon-level weighted value known as the Importance Value Index (IVI), which grades percentage values on a scale from 0 to 100 (Alanís-Rodríguez et al., 2020).
The following equation was used to estimate relative abundance (Alanís-Rodríguez et al., 2020):
Where:
AR i = Relative abundance of species i in relation to total abundance
A i = Absolute abundance of species i expressed as the number of total individuals per hectare (N ind ha-1)
i = 1,….n
Dominance was estimated by the equation (Alanís-Rodríguez et al., 2020):
Where:
DR i = Relative dominance of species i with respect to total dominance
D i = Absolute dominance of the species i (m2 ha-1)
i = 1,….n
The absolute and relative frequencies were estimated using the equations (Alanís-Rodríguez et al., 2020):
Where:
F i = Absolute frequency (percentage of presence at sampling sites)
f i = Number of sites where the species i is present
N = Number of sampling sites
FR i = Relative frequency of species i with respect to total frequency
i = 1,….n
The importance value index (IVI) was calculated with the equation (Alanís-Rodríguez et al., 2020):
The alpha diversity was determined using three indexes: the Margalef index (D Mg ), which is based on the quantification of the number of species present (specific richness); the Shannon index (H'), which refers to the numerical structure of the community ―i.e., the proportional distribution of the abundance of each species (Moreno, 2001)―, and the Shannon True Diversity index, which allows the unified and intuitive interpretation of diversity (Jost, 2006).
The following equation was used to estimate the Margalef index (D Mg ) (Clifford and Stephenson, 1975):
Where:
S = Number of species present
N ind = Total number of individuals
The Shannon Index (H) was calculated using the following formula (Shannon, 1948):
Where:
S = Number of species present
p i = n i /N ind
N ind = Total number of individuals
n i = Number of individuals of species i
Shannon's True Diversity index was estimated using the expression (Jost, 2006):
Where:
1 D = Shannon True Diversity Index
exp = Exponential
H´ = Shannon Diversity Index
The vertical structure was characterized according to the index of Vertical Distribution of Species (A) (Pretzsch, 2009), which considers three height zones or strata: zone I, with an interval of 80-100 % is the maximum height of the tree; zone II, with an interval of 50 %- 80 %; and zone III, with an interval of 0 to 50 %. This index is a modification of the Shannon index (Pretzsch, 2009), which indicates values between zero and a maximum value (A max ). A value of A max = 0 means that the stand is made up of a species that occurs in only one stratum. A max is achieved when all taxa exist in the same proportion, both in the stand and in the various strata (Pretzsch, 2009). It serves to determine the structural diversity in terms of vertical distribution of species and is calculated with the following formula:
Where:
S = Number of species present
Z = Number of height strata
p ij = Relative number of individuals of each species per area
Where:
n i,j = Number of individuals of the same species (i) in the area (j)
N = Total number of individuals
In order to compare the Pretzsch index, it was standardized by means of the value of A max , which is calculated as follows:
The value of A can then be standardized according to its relative value (A rel ), as follows:
Results
Five tree species and five shrub species belonging to nine genera and six different families were recorded. Fabaceae was the most representative, with five species (Acacia greggii A. Gray, Acacia rigidula Benth, Caesalpinia mexicana A. Gray, Ebenopsis ebano (Berland.) Barneby & J. W.Grimes, and Havardia pallens (Benth.) Britton & Rose); while the rest of the families only had one taxon (Table 1). Helietta parvifolia exhibited the highest values in abundance, dominance and frequency; it concentrated 42.66 % of the IVI, followed by Cordia boissieri A. DC. and Havardia pallens, with 17.31 and 15.83 %, respectively (Table 2).
Table 1 Scientific name, common name, family and life form of the species in the area of study (ordered by scientific name).
| Scientific name | Common name | Family | Life form |
|---|---|---|---|
| Acacia greggii A. Gray | Catclaw acacia | Fabaceae | Scrub |
| Acacia rigidula Benth | Chaparro prieto | Fabaceae | Scrub |
| Caesalpinia mexicana A. Gray | Mexican holdback | Fabaceae | Arboreal |
| Celtis pallida Torr. | Desert hackberry | Cannabaceae | Scrub |
| Cordia boissieri A. DC. | Anacahuita | Boraginaceae | Arboreal |
| Diospyros texana Scheele | Texas persimmon | Ebenaceae | Arboreal |
| Ebenopsis ebano (Berland.) Barneby & J.W.Grimes | Ebony | Fabaceae | Arboreal |
| Havardia pallens (Benth.) Britton & Rose | Tenaza | Fabaceae | Scrub |
| Helietta parvifolia A. Gray | Barreta | Rutaceae | Scrub |
| Juglans regia L. | Walnut | Juglandaceae | Arboreal |
Table 2 Structural parameters of the species registered in the study area, ordered according to their Importance Value Index (IVI).
| Species | Abundance | Dominance | Frequency | IVI | |||
|---|---|---|---|---|---|---|---|
| N ha-1 | % | m²ha-1 | % | Sites | % | ||
| Helietta parvifolia A. Gray | 1 280 | 52.24 | 11 821.65 | 54.22 | 20 | 21.51 | 42.66 |
| Cordia boissieri A. DC. | 335 | 13.67 | 4 119.33 | 18.89 | 18 | 19.35 | 17.31 |
| Havardia pallens (Benth.) Britton & Rose | 375 | 15.31 | 2 564.19 | 11.76 | 19 | 20.43 | 15.83 |
| Junglas regia L. | 215 | 8.78 | 1 304.01 | 5.98 | 13 | 13.98 | 9.58 |
| Ebenopsis ébano (Berland.) Barneby & J.W.Grimes | 110 | 4.49 | 418.50 | 1.92 | 8 | 8.60 | 5.00 |
| Acacia rigidula Benth | 65 | 2.65 | 901.42 | 4.13 | 5 | 5.38 | 4.05 |
| Diospyros texana Scheele | 35 | 1.43 | 486.08 | 2.23 | 4 | 4.30 | 2.65 |
| Caesalpinia mexicana A. Gray | 20 | 0.82 | 107.15 | 0.49 | 3 | 3.23 | 1.51 |
| Acacia greggii A. Gray | 10 | 0.41 | 53.57 | 0.25 | 2 | 2.15 | 0.93 |
| Celtis palida Torr. | 5 | 0.20 | 26.65 | 0.12 | 1 | 1.08 | 0.47 |
| Total | 2 450 | 100 | 2 1803 | 100 | 93 | 100 | 100 |
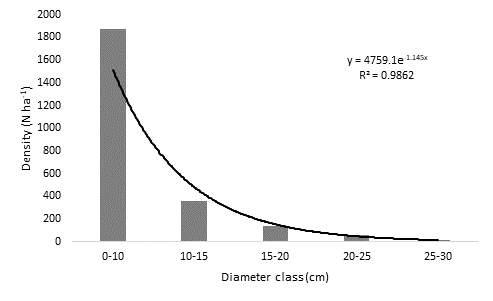
The density of individuals per hectare showed a negative exponential trend line as the diameter increased; the 0-10 cm diameter class exhibited the highest density of individuals, with values above 1 800 N ha-1 (Figure 2).
Helietta parvifolia had the highest Importance Value Index (Table 2); in addition, the same negative exponential trend was observed in the density of individuals, and for the 0-10 cm diameter class the highest value registered, was above 800 N ha-1 (Figure 3).

Figure 3 Density of individuals according to the diameter classes in the study area for Helietta parvifolia A. Gray.
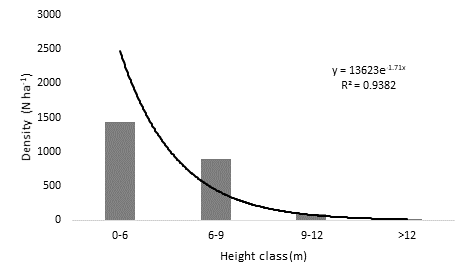
The total height of the individuals varied between 2 and 12 m. The highest abundance of individuals corresponded to the 0 - 6 m height class, with 1 440 N ha-1; in the higher height classes, a negative trend in the density of trees was observed as the height increased (Figure 4). Individuals with a height below or equal to 9 m amounted to 95 % of the total measured.
In the plant community under study, Margalef index values of 1.45, Shannon index values of 1.47, and True Shannon Diversity index values of 4.34 were estimated. In relation to the Vertical Index of Species (A), a medium structural diversity was observed in the high strata, with a value of 2.16, an A max of 3.40 and an A rel of 63.72 %; in the third stratum, 62.8 % of the evaluated individuals were registered.
As for the analysis of vertical distribution between the high, medium and low strata, the high stratum was formed by Helietta parvifolia and Havardia pallens, with 40 and 10 N ha-1, respectively, representing 2 % of the total. The middle stratum is made-up by Helietta parvifolia, Havardia pallens, Juglans regia L., Ebenopsis ebano, Acacia rigidula, Diospyros texana Scheele and Cordia boissieri, with 535, 170, 25, 10, 25, 5, and 90 N ha-1, respectively, i.e., 35 % of the total in the study area (Table 3). Helietta parvifolia, Cordia boissieri, Havardia pallens, Juglans regia, Diospyros texana, Ebenopsis ebano, Caesalpinia mexicana, Acacia rigidula, and Celtis pallida Torr. were recor/ded in the low stratum, with 705, 245, 195, 190, 30, 100, 20, 10, 35, and 5 N ha-1, respectively, amounting to 63 % of the counted individuals.
Table 3 Abundance values (N ha-1), Vertical Pretzsch Index (A), A max and A rel . The three layers are shown: High (I), Medium (II) and Low (III) stratum.
| Stratum | Species | N |
|
Pretzsch index | % | ||
|---|---|---|---|---|---|---|---|
|
|
Ln |
|
|||||
| I | Helietta parvifolia A. Gray | 8 | 40 | 0.016 | -4.112 | -0.067 | 2 |
| Havardia pallens (Benth.) Britton & Rose | 2 | 10 | 0.004 | -5.499 | -0.022 | ||
| Total | 10 | 50 | 0.020 | -9.612 | -0.089 | ||
| II | Helietta parvifolia A. Gray | 107 | 535 | 0.218 | -1.519 | -0.332 | 35 |
| Havardia pallens (Benth.) Britton & Rose | 34 | 170 | 0.069 | -2.666 | -0.185 | ||
| Juglans regia L. | 5 | 25 | 0.010 | -4.582 | -0.046 | ||
| Ebenopsis ébano (Berland.) Barneby & J.W.Grimes | 2 | 10 | 0.004 | -5.499 | -0.022 | ||
| Acacia rigidula Benth | 5 | 25 | 0.010 | -4.582 | -0.046 | ||
| Diospyros texana Scheele | 1 | 5 | 0.002 | -6.192 | -0.012 | ||
| Cordia boissieri A. DC. | 18 | 90 | 0.036 | -3.301 | -0.121 | ||
| Total | 172 | 860 | 0.351 | -28.344 | -0.768 | ||
| III | Helietta parvifolia A. Gray | 141 | 705 | 0.288 | -1.243 | -0.358 | 63 |
| Cordia boissieri A. DC. | 49 | 245 | 0.100 | -2.300 | -0.230 | ||
| Havardia pallens (Benth.) Britton & Rose | 39 | 195 | 0.079 | -2.528 | -0.201 | ||
| Juglans regia L. | 38 | 190 | 0.077 | -2.554 | -0.198 | ||
| Diospyros texana Scheele | 6 | 30 | 0.012 | -4.400 | -0.053 | ||
| Ebenopsis ebano (Berland.) Barneby & J.W.Grimes | 20 | 100 | 0.040 | -3.196 | -0.130 | ||
| Caesalpinia mexicana A. Gray | 4 | 20 | 0.008 | -4.806 | -0.039 | ||
| Acacia greggii v | 2 | 10 | 0.004 | -5.499 | -0.022 | ||
| Acacia rigidula Benth | 7 | 35 | 0.014 | -4.246 | -0.060 | ||
| Celtis pallida Torr. | 1 | 5 | 0.002 | -6.192 | -0.012 | ||
| Total | 307 | 1 535 | 0.627 | -36.969 | -1.309 | ||
| Total | 489 | 2 445 | 1 | -74.925 | -2.166 | 100 | |
| A | 2.167 | ||||||
| A max | 3.401 | ||||||
| A rel | 63.723 | ||||||
Discussion
The Fabaceae family was the best represented, a result that agrees with what has been observed in several researches carried out on both mature and regenerated submontane scrub plant communities (García-Hernández and Jurado, 2008; Canizales-Velázquez et al., 2009; Estrada-Castillón et al., 2012; Alanís-Rodríguez et al., 2015; Canizales-Velázquez et al., 2015; Mora-Olivo et al., 2016). This family is diverse and well represented in the different plant communities of northern Mexico, given their climatic affinity and the presence of different topographic and soil conditions of the region (Estrada et al., 2005). The dominance of Fabaceae over other groups is due to their ability to fix nitrogen in the soil. This is because, in arid and semi-arid environments, nitrogen is the most limiting element of all soil nutrients required for plant development and growth, as well as an essential element for microbial growth and for the degradation of organic matter (Ferrara and Alarcón, 2001; Celaya-Michel and Castellanos-Villegas, 2011).
Helietta parvifolia exhibited the highest Importance Value Index (IVI), with 42.66 %. This species is also recorded by García-Hernández and Jurado (2008) with the highest IVI (25 %) in a shrub located in Linares, Nuevo León, Mexico; H. parvifolia, together with Cordia boissieri, Havardia pallens, Acacia rigidula, Diospyros texana, and Celtis pallida made up 79.41 % of the Value of Importance of that community. This pattern of composition and dominance strengthens the observation of Foroughbakhch and Ngangyo (2017), who suggest that a community dominated by the above species is, from a structural point of view, a high thornscrub.
Estrada-Castillón et al. (2012) indicate that, among the xerophilous scrubs, the submontane scrub exhibits different physiognomic characteristics that divide it into high, medium and low; the first includes the association dominated by H. parvifolia. Meléndez-Jaramillo et al. (2019) indicate that H. parvifolia is one of the species with the greatest presence in the submontane scrub of northeastern Mexico that is located at an altitudinal range of 500 to 800 m.
Most individuals of Helietta parvifolia were found in the smaller diameter categories; of these, 0-10 cm concentrated the largest number of individuals, and a minority of individuals exceeded 15 cm in diameter. In the height classes, the corresponding 0 - 6 m included the largest number of individuals, and the smallest number occurred in the class above 12 m. This agrees with the observations of the study in southeastern Nuevo León by Foroughbakhch et al. (2003), who recorded individuals with a diameter equal to or below 15 cm and heights ranging between 3 and 12 m. These findings agree with the vertical characterization of the submontane scrub, where Helietta parvifolia reached a maximum height of 6 m, as reported by García-Hernández and Jurado (2008).
The presence in the community under study of a high number of individuals (1 800 N ha-1) belonging to the diameter class of 0 - 10 cm suggests the existence of an active regenerative community, composed mainly of saplings and young trees. Of this regenerative community, 47.7 % of them belonged to H. parvifolia, which had the highest Importance Value Index, was dominant in the upper stratum, and contributed more than 83 % of the individuals in the diameter classes over 20 cm.
H. parvifolia is a slow-growing, high-density wood taxon (Foroughbakhch et al., 2011), whose seeds have dormancy as well as independence from direct light for germination (Jurado et al., 2000, 2001). All these characteristics indicate that H. parvifolia is long-lived and has some tolerance to shade, which explains its dominance in the regenerative community under the canopy of this mature plant community. The observation is in line with that made by García-Hernández and Jurado (2008), in the sense that H. parvifolia is part of a mature or undisturbed vegetation.
Furthermore, the dominance of H. parvifolia in this study also suggests a relationship with at least two other factors: the allelopathic characteristics of H. parvifolia (Graue, 1981) ―a product of the alkaloid-type substances in its leaves (coumarins of the furan-quinoline type) that can inhibit the germination and growth of those plants that come into contact with them (Graue, 1981; Gómez-Calvario et al., 2019)― and the history of land use in the site, which has no record of disturbances, previous clearings due to forestry activities, or exploitation of timber species like H. parvifolia, and has only been used for the collection of edible fruits and woody material from the ground.
Both the lower importance value indexes of the taxa typical of the first years of bush recovery (pioneers and long-lived pioneers such as Celtis pallida, Acacia greggi, Acacia rigidula) and the higher importance values of species with characteristics of mature vegetation organisms (increased life spectrum and greater tolerance to shade, such as Cordia boissieri, H. parvifolia) coincide with the site land-use history information, which suggests a mature scrub, as well as with the observations made by García-Hernández and Jurado (2008) regarding the composition of the bushes under pristine conditions.
The evaluated area had Margalef index values of 1.45 and Shannon index values of 1.47. The former is similar to other scrublands in northeastern Mexico; for example, the Tamaulipan thornscrub (Pequeño-Ledezma et al., 2012; Jiménez et al., 2012, Mora et al., 2013a), but lower than that cited by Alanís-Rodríguez et al. (2015): D Mg = 6.02; and that of Canizales-Velázquez (2009): D Mg = 6.02. The Shannon entropy index is low compared to the Tamaulipan thornscrub (Pequeño Ledezma et al., 2012; Jiménez et al., 2012, Mora et al. 2013b); lower than that indicated by Alanís-Rodríguez et al. (2015), D Mg = 3.02 and Canizales-Velázquez (2009), D Mg = 3.00. These differences are attributable to the fact that Alanís-Rodríguez et al. (2015) and Canizales-Velázquez (2009) evaluated submontane scrublands at higher altitudes and recorded a high number of species typical of Quercus forests.
The Vertical Index of Species (A) was 2.16, with an A max of 3.40 and an A rel of 63.7 %, signifying an average structural diversity in the altitude strata. A rel values close to 100 % indicate that all species are equally distributed in the three height strata (Pretzsch, 2009). These results are similar to those obtained in researches developed in thornscrub communities, by Jiménez et al. (2009), Mora-Donjuán et al. (2014), and Pequeño Ledezma et al. (2012), who obtained an A max of 4.56, 3.08 and 2.2, and an A rel of 67.1 %, 53.8 % and 63.7 %, respectively. The Vertical Index (A) has not been estimated for other submontane scrublands. Based on the estimated value of A, it may be said that the vegetal communities of the Tamaulipan thornscrub and the submontane scrubs show similarity in relation to the Vertical Index of Species. They both exhibit a well-defined vertical structure, where strata II and III (medium and low), which concentrate the largest amount of species and abundances (N ha-1), are the most prominent.
Conclusions
The studied community exhibits similar values of species richness and diversity in comparison with other plant associations of arid and semi-arid climate in Northeast Mexico. Diameter and height class graphs for the plant community and, specifically, for Helietta parvifolia show an inverted jack, which indicates the existence of an active regenerative community, composed mainly of saplings and young trees. The most important family, due to its contribution to the community, is Fabaceae; and the most important species are Helietta parvifolia, Cordia boissieri, and Havardia pallens.
Referencias
Alanís-Rodríguez, E., J. Jiménez-Pérez, A. Mora-Olivo, J. G. Martínez-Ávalos, J. M. Mata-Balderas, A. C. Collantes-Chávez-Costa, y E. A. Rubio-Camacho. 2015. Estructura y diversidad del matorral submontano contiguo al área metropolitana de Monterrey, Nuevo León, México. Acta Botánica Mexicana (113): 1-19. Doi: 10.21829/abm113.2015.1093. [ Links ]
Alanís-Rodríguez, E. , A. Mora-Olivo y J. S. Marroquín de la F. 2020. Muestreo ecológico de la vegetación. Editorial Universitaria de la Universidad Autónoma de Nuevo León. Monterrey, N.L., México. 245 p. https://www.researchgate.net/publication/343137042_Muestreo_Ecologico_de_la_vegetacion (12 de junio de 2020). [ Links ]
Canizales-Velázquez, P. A., O. A. Aguirre-Calderón, E. Alanís-Rodríguez, E. J. Treviño-Garza and J. M. Mata-Balderas . 2015. Structural analysis of shrublands adjacent to the Metropolitan Area of Monterrey, Mexico. Journal of the Botanical Research Institute of Texas 9(1):173-185. Doi: 10.2307/24621262. [ Links ]
Canizales-Velázquez, P. A. , E. Alanís-Rodríguez , R. Aranda-Ramos, J. M. Mata-Balderas , J. Jiménez-Pérez , G. Alanís-Flores y M. G. Ruiz-Bautista. 2009. Caracterización estructural del matorral submontano de la Sierra Madre Oriental, Nuevo León. Revista Chapingo Serie Ciencias Forestales y Del Ambiente. 15(2): 115-120. https://revistas.chapingo.mx/forestales/?section=articles&subsec=issues&numero=40&articulo=516 (10 de septiembre de 2020). [ Links ]
Carrillo, A., R. Foroughbachk, V. Bustamante, C. Wehenkel and H. González. 2013. Natural durability of wood of ten native species from northeastern Mexico. Forest Science and Practice. 15(2): 160-166. Doi: 10.1007/s11632-013-0201-2. [ Links ]
Celaya-Michel, H., y A. E. Castellanos-Villegas. 2011. Mineralización de nitrógeno en el suelo de zonas áridas y semiáridas. Terra Latinoamericana 29(3): 343-356. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-57792011000300343&lng=es&tlng=es (4 de mayo de 2020). [ Links ]
Clifford, H. T. and W. Stephenson. 1975. An introduction to numerical classification. London Academic Press. New York, NY, USA. 229 p. [ Links ]
Estrada-Castillón, E., J. Á. Villarreal-Quintanilla, E. Jurado-Ybarra, C. Cantú-Ayala, M. A. García-Aranda, J. Sánchez-Salas, J. Jiménez-Pérez y M. Pando-Moreno. 2012. Clasificación, estructura y diversidad del matorral submontano adyacente a la planicie costera del Golfo Norte en el noreste de México. Botanical Science 90(1): 37-52. Doi: 10.17129/botsci.384. [ Links ]
Estrada C., E., J. Á. Villarreal Q. y E. Jurado. 2005. Leguminosas del norte del estado de Nuevo León, México. Acta botánica mexicana 73: 1-18. Doi: 10.21829/abm73.2005.1003. [ Links ]
Ferrara C., R. F. y A. Alarcón. 2001. La microbiología del suelo en la agricultura sostenible. CIENCIA ergo-sum, Revista Científica Multidisciplinaria de Prospectiva, 8(2): 175-183. https://www.redalyc.org/pdf/104/10402108.pdf (10 de septiembre de 2020). [ Links ]
Foroughbakhch, F. and N. H. Ngangyo. 2017. Structure and Floristic Compositions of Ecosystems of Northeast of Mexico. Chronicle of Bioresource Management 1(2):60-64. https://www.pphouse.org/cbm-article-details.php?cbm_article=14 (21 de mayo de 2020). [ Links ]
Foroughbakhch, R. 1992. Establishment and growth potential of fuelwood species in northeastern Mexico. Agroforestry Systems. 19(2): 95-108. Doi: 10.1007/BF00138500. [ Links ]
Foroughbakhch, R., M. A. Alvarado-Vázquez, A. Núñez-González, J. Hernández-Piñero and A. Rocha-Estrada 2003. Structural analysis and performance of Helietta parvifolia (Gray) Benth in southeastern Nuevo Leon, Mexico. Interciencia. 28(11): 651-655. https://www.redalyc.org/articulo.oa?id=339/33908605 (4 de mayo de 2020). [ Links ]
García-Hernández, J. y E. Jurado. 2008. Caracterización del matorral con condiciones prístinas en Linares NL, México. Ra Ximhai: Revista Científica de Sociedad, Cultura y Desarrollo Sostenible. 4(1): 1-22. https://dialnet.unirioja.es/servlet/articulo?codigo=2575952 (4 de mayo de 2020). [ Links ]
Gómez-Calvario, V., M. Á. Ramírez-Cisneros, M. Acevedo-Quiroz and M. Y. Rios. 2019. Chemical composition of Helietta parvifolia and its in vitro anticholinesterase activity. Natural product research 33(6): 889-892. Doi:10.1080/14786419.2017.1410808. [ Links ]
González-Medrano, F. 2012. Las zonas áridas y semiáridas de México y su vegetación. Secretaría de Medio Ambiente y Recursos Naturales. México, D.F., México. 173 p. http://www2.inecc.gob.mx/publicaciones2/libros/668/zonas.pdf (18 de mayo de 2020). [ Links ]
Graue W., C. B. 1981. Estudio del Potencial Inhibidor y Alelopático de Helietta Parvifolia (Gray) Benth. Especie del Matorral Submontano de Nuevo León, México. Tesis de Maestría en Ciencias con Especialidad Agrícola. Instituto Tecnológico y de Estudios Superiores de Monterrey. Monterrey, N.L., México. 86 p. https://repositorio.tec.mx/handle/11285/569322 (6 de junio de 2020). [ Links ]
Hooper, D. U., F. S. Chapin III, J. J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J. H. Lawton, D. M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setälä, A. J. Symstad, J. Vandermeer and D. A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological monographs 75(1): 3-35. Doi: 10.1890/04-0922. [ Links ]
Instituto Nacional de Estadística Geografía e Informática (INEGI). 2009. Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Municipio de Linares, Nuevo León, Clave geoestadística 19033. http://www3.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/19/19033.pdf (4 de mayo de 2020). [ Links ]
Jiménez J., E. Alanís, O. A. Aguirre, M. Pando y M. A. González. 2009. Análisis sobre el efecto del uso del suelo en la diversidad estructural del matorral espinoso tamaulipeco. Madera y Bosques. 15: 5-20. Doi: 10.21829/myb.2009.1531183. [ Links ]
Jiménez, J., E. Alanís , J. L. Ruiz, M. A. González, J. I. Yerena y G. J. Alanís. 2012. Diversidad de la regeneración leñosa del matorral espinoso tamaulipeco con historial agrícola en el NE de México. Ciencia UANL. 15(2): 66-71. http://eprints.uanl.mx/2995/1/12ArticuloLenos.pdf (4 de mayo de 2020). [ Links ]
Jost, L. 2006. Entropy and diversity. Oikos 113:363-375. Doi:10.1111/j.2006.0030-1299.14714.x. [ Links ]
Jurado, E., O. A. Aguirre , J. Flores, J de J. Navar, H. Villalón and D. Wester. 2000. Germination in tamaulipan thornscrub of north-eastern Mexico. Journal of Arid Environments 46(4): 413-424. Doi: 10.1006/jare.2000.0684. [ Links ]
Jurado, E. , J de J. Navar, H. Villalón and M. Pando . 2001. Germination associated with season and sunlight for Tamaulipan thornscrub plants in north-eastern Mexico. Journal of arid environments 49(4): 833-841. Doi: 10.1006/jare.2001.0817. [ Links ]
Maginot, N. H., P. R. Foroughbakhch, A. Carrillo-Parra and L. R. Salas Cruz. 2014. Estimation of timber production of five species of the tamaulipas thorny shrubs growing in native stands and plantations. Open Journal of Forestry 4: 239-248. Doi: 10.4236/ojf.2014.43031. [ Links ]
Meléndez-Jaramillo, E., C. Cantú-Ayala , U. J. Sánchez-Reyes, F. M. Sandoval-Becerra and B. Herrera-Fernández. 2019. Altitudinal and seasonal distribution of butterflies (Lepidoptera, Papilionoidea) in Cerro Bufa El Diente, Tamaulipas, Mexico. ZooKeys 900: 31-68. Doi: 10.3897/zookeys.900.36978. [ Links ]
Molina-Guerra, V. M., A. Mora-Olivo , E. Alanís -Rodríguez , B. Soto-Mata y A. M. Patiño-Flores. 2019. Plantas características del matorral espinoso tamaulipeco en México. Editorial Universitaria de la Universidad Autónoma de Nuevo León. Monterrey, N.L., México. 114 p. https://www.academia.edu/42036384/Plantas_caracter%C3%ADsticas_del_matorral_espinoso_tamaulipeco_en_M%C3%A9xico (15 de abril de 2020). [ Links ]
Montagnini, F. and C. Jordan. 2005. Characteristhics of tropical forest. In: Montagnini, F. and C. Jordan . (Eds.). Tropical forest management, the basis for conservation and management. Springer. Berlin, Heidelberg, The Netherlands. pp. 19. [ Links ]
Mora D., C. A., E. Alanís R. , J. Jiménez P., M. A. González T., J. I. Yerena Y. y L. G. Cuellar R. 2013a. Estructura, composición florística y diversidad del matorral espinoso tamaulipeco, México. Ecología Aplicada 12(1): 29-34. Doi: 10.21704/rea.v12i1-2.435. [ Links ]
Mora D., C. A. , J. Jiménez P., E. Alanís R., E. A. Rubio C., J. I. Yerena Y. y M. A. González T. 2013b. Efecto de la ganadería en la composición y diversidad arbórea y arbustiva del matorral espinoso tamaulipeco. Revista Mexicana de Ciencias Forestales 4(17): 124-137. Doi: https://doi.org/10.29298/rmcf.v4i17.426 [ Links ]
Mora-Donjuán, C. A., E. A. Rubio-Camacho , E. Alanís -Rodríguez , J. Jiménez-Pérez , M. A. González-Tagle, J. M. Mata-Balderas y A. Mora-Olivo . 2014. Composición y diversidad vegetal de un área de matorral desértico micrófilo con historial pecuario en el noreste de México. Polibotánica. (38): 53-66. https://www.polibotanica.mx/ojs/index.php/polibotanica/article/view/378 (21 de mayo de 2020). [ Links ]
Mora-Olivo, A., E. Alanís-Rodrígue , J. J. Marroquín-Castillo, T. I. Sarmiento-Muñoz, J. G. Martínez-Ávalos , F. Garza-Ocañas and J. A. Torres-Castillo. 2016. Structure and diversity of a submontane scrub community in Tamaulipas, Mexico. Interciencia 41(11): 769-773. https://www.interciencia.net/wp-content/uploads/2017/10/769-MORA-41-11.pdf (21 de mayo de 2020). [ Links ]
Moreno, C. E. 2001. Métodos para medir la biodiversidad. M&T-Manuales y Tesis SEA. Vol. 1. Zaragoza, España. 84 p. http://tuxchi.iztacala.unam.mx/disweb/demo_ecologia/pdfs/libros/mantes1.pdf (10 de mayo de 2020). [ Links ]
Návar, J., J. Nájera and E. Jurado . 2001. Preliminary estimates of biomass growth in the Tamaulipan thornscrub in north-eastern Mexico. Journal of Arid Environments 47(3): 281-290. Doi: 10.1006/jare.2000.0708. [ Links ]
Pequeño-Ledezma, M. A., E. Alanís-Rodríguez, J. Jiménez-Pérez, M. A. González-Tagle, J. I. Yerena-Yamallel, L. G. Cuellar-Rodríguez y A. Mora-Olivo . 2012. Análisis de la restauración pasiva post-pecuaria en el matorral espinoso tamaulipeco del noreste de México. CienciaUAT. 7(1): 48-53. Doi: 10.29059/cienciauat.v7i1.39. [ Links ]
Pretzsch, H. 2009. Forest Dynamics, Growth and Yield. From Measurement to Model. Springer-Verlag. Berlín Heidelberg, Alemania. 664p. [ Links ]
Rojas-Mendoza, P. 1965. Generalidades sobre la vegetación del estado de Nuevo León y datos acerca de su flora. Tesis doctoral. Facultad de Ciencias, Universidad Nacional Autónoma de México. México, D.F., México. 125 p. [ Links ]
Rzedowski, J. 1978. Vegetación de México. Editorial Limusa. México, D.F. México. 745 p. [ Links ]
Rzedowski, J. 1991. Diversidad y orígenes de la flora fanerogámica de México. Acta Botánica Mexicana (14): 3-21. Doi: 10.21829/abm14.1991.611. [ Links ]
Santos, T. y J. L. Tellería. 2006. Pérdida y fragmentación del hábitat: efecto sobre la conservación de las especies. Ecosistemas 15 (2): 3-12. https://revistaecosistemas.net/index.php/ecosistemas/article/view/180 (10 de septiembre de 2020). [ Links ]
Secretaría de Medio Ambiente y Recursos Naturales (Semarnat). 2006. El Medio Ambiente en México 2005: en resumen. México, D.F., México. 91 p. [ Links ]
Shannon, C. 1948. The mathematical theory of communication. In: Shannon, C. E. and W. Weaver (Eds.). University of Illinois Press. Champaign, IL, USA. 134-154. [ Links ]
Uvalle-Sauceda, J. I., L. Reséndiz-Dávila, F. F. González-Saldívar y C. M. Cantú-Ayala. 2015. Caracterización de la vegetación de matorral submontano relacionada con capacidad de carga animal. Agroproductividad 8(5): 42-48. https://www.researchgate.net/profile/Cesar_Cortez-Romero/publication/289539472_PHYLOGEOGRAPHY_APPLIED_TO_THE_CONSERVATION_OF_WILD_FAUNA_REVIEW_AND_RESULTS_-_FILOGEOGRAFIA_APLICADA_EN_LA_CONSERVACION_DE_FAUNA_SILVESTRE_REVISION_Y_RESULTADOS/links/56900a9c08aee91f69a14678.pdf#page=44 (10 de septiembre de 2020). [ Links ]
Received: May 05, 2020; Accepted: October 20, 2020











 texto em
texto em