Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.10 no.56 México nov./dic. 2019 Epub 30-Abr-2019
https://doi.org/10.29298/rmcf.v10i56.545
Scientific article
In vitro adventitious shoot morphogenesis of the Mexican Douglas-fir Pseudotsuga menziesii (Mirb.) Franco
1Cenid-Comef, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. México.
2Instituto Tecnológico Superior de San Martín Texmelucan. México.
3Centro de Investigación en Ciencias Biológicas, Universidad Autónoma de Tlaxcala. México.
4Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad en México. México.
5Laboratorio de Cultivo de Tejidos Vegetales Jardín Botánico del Instituto de Biología, Universidad Nacional Autónoma de México. México.
6Departamento de Biología Comparada, Laboratorio de Desarrollo en Plantas, Facultad de Ciencias, Universidad Nacional Autónoma de México. México.
Pseudotsuga menziesii is a conifer of ecological and economic importance worldwide. Due to the small size of its native populations in Mexico, natural regeneration in this species is poor, therefore in vitro cultivation is considered as an alternative strategy for its propagation and conservation. The effect of two combinations of auxins and cytokinins in the induction of adventitious shoots was compared. Mature embryos were grown on solid Schenk and Hildebrandt (SH) medium containing 0.53/13.32 μM of 1-naphthalene acetic acid (NAA) / 6-benzyladenine (BA) or 2,4-dichlorophenoxyacetic acid (2,4-D) / kinetin (K). The buds were formed via direct organogenesis in the hypocotyl and cotyledons. After 90 and 130 days of culture, the organogenic capacity was higher in NAA / BA and extended for a longer period than in 2,4-D / K, although the latter the shoots were more vigorous. Histological analysis showed the sequential appearance of distinct promeristemoids and meristemoides on the surface far from the medium, whereas wide meristematic zones and meristematic nodules formed in the tissues in contact with the medium, which gave rise to the outbreaks, was detected in both treatments. Because the buds formed included cells of the epidermis and embryonic subepidermis a multicellular origin is proposed. This work allowed us to better understand the events associated with direct organogenesis that may impact on the consolidation of regeneration protocols and future improvement strategies.
Key words Auxins; caulogenesis; ciokinines; tissue culture; ontogeny; Pseudotsuga
Pseudotsuga menziesii es una conífera de importancia ecológica y económica a nivel mundial. Debido al reducido tamaño de sus poblaciones nativas en México, la especie enfrenta serias dificultades para su regeneración natural, por lo que el cultivo in vitro se plantea como estrategia alternativa para su propagación y conservación. Se comparó el efecto de dos combinaciones de auxinas y citocininas en la inducción de brotes adventicios. Se cultivaron embriones maduros en medio Schenk y Hildebrandt (SH) adicionado con 0.53/13.32 M de ácido 1-naftalenacético (ANA)/ 6-benciladenina (BA) o ácido 2,4-diclorofenoxiacético (2,4-D) / cinetina (K). Los brotes se formaron vía organogénesis directa en el hipocotilo y en cotiledones. Después de los 90 y 130 días de cultivo, la capacidad organogénica fue mayor en ANA/BA y se extendió por un período más largo que en 2,4-D/K, aunque este último los brotes fueron más vigorosos. Mediante un análisis histológico se detectó, en ambos tratamientos, la aparición secuencial de promeristemoides, meristemoides en la superficie alejada del medio, así como amplias zonas meristemáticas y nódulos meristemáticos en los tejidos en contacto con el medio, que dieron lugar a los brotes. Debido a que los brotes formados incluyeron células de la epidermis y subepidermis embrionaria se propone un origen multicelular. Este trabajo permitió comprender mejor los eventos asociados a la organogénesis directa que pueden impactar en la consolidación de los protocolos de regeneración y futuras estrategias de mejoramiento.
Palabras clave Auxinas; caulogénesis; citocininas; cultivo de tejidos; ontogenia; Pseudotsuga
Introduction
Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), known in Mexico as “pinabete” is an ecologically and economically important conifer that grows from British Columbia in Canada to central Mexico (Hermann and Lavender 1990; Gugger et al., 2011). Populations of P. menziesii in Mexico make up the southernmost limit of its natural range, and are found in small, fragmented stands (Vargas et al., 2004). Additionally to its disjunct distribution, loss of forest land to agriculture and grazing, has decreased the number of individuals participating in reproduction, resulting in low natural regeneration, due to reduced seed production and quality, seedling recruitment, as well as high levels of endogamy in many populations, particularly towards the extreme southern limit of its distribution, in the states of Hidalgo, Tlaxcala, Puebla and Oaxaca (Mápula et al., 1996, Zavala and Méndez, 1996, Juárez et al., 2006, Velasco et al., 2007). Hence, it has been listed as a species subject to special protection by the Official Mexican Standard (NOM-059-SEMARNAT-2010), and considered as a priority species for conservation and reforestation planning (Vargas et al., 2004), to promote strategies that favor its regeneration, as well as alternatives for sustainable use.
The in vitro culture of plant tissues offers important perspectives for the propagation of forest species. The techniques represent the basis for research in propagation, improvement and germplasm conservation. However, for conifers it remains complicated and success is often limited to particular genotypes within species (Bonga, 2015). The in vitro propagation of P. menziesii has been achieved through the use of explants originated from native populations of the United States of America; several protocols have been obtained for the regeneration of adventitious shoots from embryo and cotyledon culture, through direct and indirect organogenesis (Cheng and Voqui, 1977, Winton and Verhagen, 1977, Cheah and Cheng, 1978), as well as the proliferation and lengthening of apical and axillary buds (Gupta and Durzan, 1985) and somatic embryos (Durzan and Gupta, 1987).
However, it has not been possible to transfer these protocols for the in vitro propagation of genotypes of Mexican provenance, and the success to date has been very limited. Using mature zygotic embryos from seeds obtained from two natural populations in Mexico (Tlaxco and Terrenate, in the Tlaxcala state), regeneration of adventitious shoots was accomplished by using twelve combinations of the growth regulators 6-benzyladenine (BA) with naphthaleneacetic acid (NAA) and kinetin (K) with 2,4-dichlorophenoxyacetic acid (2,4-D), in concentrations that ranged from 0 to 0.5 mgL-1 of auxins, and from 1 to 5 mg L-1 of cytokinins. Rooting was sporadic, which restricted the implementation of this propagation technology on a larger scale (Galindo et al., 2000). Subsequently, callus formation and embryonic-type structures were obtained through mature zygotic embryo culture; although the authors did not show histological evidence, nor the development of seedlings (Carrillo et al., 2011). The variability of the morphogenetic response shows that very little is known about how the different factors involved in the culture interact and influence the morphogenetic response of the explants, particularly with regard to the nature of the growth regulators.
From the interest in consolidating an in vitro propagation system that allows the regeneration of Mexican genotypes of P. menziesii, in the present work the description and comparison of the morphogenetic response of mature zygotic embryos exposed to two combinations of growth regulators was sought; the ability to induce adventitious buds was assessed, as well as the pattern of their development with respect to the type of tissue involved and the effect of their distance with the culture medium. Through a structural analysis it was possible to deepen the understanding of the origin and cellular events that occur prior to the differentiation of the shoot buds, and which serve to explain the effect that growth regulators have on maintaining the organogenic ability.
Materials and Methods
Provenance of the biologic material
200 mature seeds of P. menziesii donated by the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (National Institute of Forestry, Agriculture and Livestock Research) (INIFAP) were used. This material was collected in Terrenate, Tlaxcala forests from an undetermined number of trees in 1996. They were stored in plastic bags in a cold room at 4 °C until.
Pretreatment of seeds
The seeds were cleaned and disinfected under aseptic conditions by immersion in: 1) 30 % H2O2 (v / v) for 60 min; 2) Benomyl (0.5 g L-1) for 24 h; Chloramphenicol (20 mg L-1) for 45 min; 3) commercial chlorine at 15 % (v / v) (6 % NaOCl) for 60 min; 4) in 1 % H2O2 (v / v) for 48 h. Between each step, the seeds were rinsed three times with plenty of sterile distilled water. Under aseptic conditions, the seminal cover was removed and as well as the megagametophytes; the latter were rinsed with a 15 % (v / v) commercial chlorine solution for 60 seconds, followed by three rinses in sterile distilled water, before dissecting the embryo. Prior to sowing the embryos, they were immersed in a chloramphenicol solution (60 mg L-1).
Induction of adventitious buds
This procedure was based on the protocol described by Galindo et al. (2000). SH medium (Schenk and Hildebrandt, 1972) supplemented with sucrose (30 g L-1), solidified with PhytagelTM (0.6%) and with one of two combinations of growth regulators: NAA /BA and 2,4-D / K to 0.53 / 13.32 µM was used. The pH of the medium was adjusted to 5.7-5.8, before sterilizing at 1.5 kg / cm2, 121 °C, 15 min, in a FE-399 Felisa sterilizer. The mature zygotic embryos were placed horizontally in Petri dishes and incubated for 30 days in plant a growth chamber (3740 Forma Scientific), at 23 ± 1 °C, with a 16 h -light and 8 h -darkness photoperiod, and PPFD of 46-48 µmolm-2s-1. For both treatments, three replicates of ten embryos per Petri dish were used.
Development and elongation of adventitious shoots
After the induction period, the embryos were transferred to an elongation medium without growth regulators, which consisted of SH medium reduced to 50 % of its concentration of inorganic components, supplemented with sucrose (3 g L-1), polyvinylpyrrolidone (PVP) (250 mg L-1), anti-vitrifying agent (0.5 g L-1) (SIGMA) and was solidified with PhytagelTM (0.6 %). They were incubated under the above-mentioned conditions. Biweekly subcultures were carried out for six months and the shoots were individualized once they exceeded a length of 0.5 mm, and subcultured onto the same medium.
Structural Analysis
For descriptive purposes, the complete mature embryo and its horizontal position on the culture media was taken as a refence, and the following regions were defined: embryo surface in direct contact with the media (SCM), embryo surface furthest away from the growth media (SAM), cotyledons (cot), el hipocotyl (hcot), apical meristem and root cap.
Five to 10 embryos from each treatment were analyzed at 0, 3, 6, 10, 12, 14, 16, 22, 30, 45 and 90 days of cultivation,; they were fixed in FAA (formaldehyde: acetic acid: alcohol: distilled water, 10:5:50:35) for a minimum of 48 h. Subsequently, the material was rinsed with distilled water and dehydrated gradually in a series of dilutions of ethyl alcohol (at 30, 50, 70, 85, 96 and 100 %), for one hour each.
For infiltration ParaplastTM infiltration, the samples were immersed in xylol for 10 min and then gradually transferred to mixtures of xylol-Paraplast in proportions of 3:1,1:1 and 1:3, for one hour each. Finally, they were placed in pure ParaplastTM for a minimum of 24 h at 56 °C, and then included. The tissue was sectioned at 6-8 μm with a rotating microtome (820 American Optical) and then stained with fast green safranin.
Data analysis
The number of shoot buds per embryo and shoot buds formed in each embryo surface were evaluated at 90 and 130 days. The data were analyzed using a GLM with a quasi Poisson family distribution. For the GLM the 'glm' function was used. The Tukey Post-hoc analysis for the GLM models was done with the 'glht' function of the 'multcomp' package. All the analysis were done through the program R 2.14.0 (R Development Core Team, 2011).
Results
When evaluating the response of the explants to the two treatments, no differences were found in the number of embryos that responded (P <0.05): in NAA / BA, 75.8 % formed shoot buds, while 73.7 % did so in 2,4-D / K (Table 1). For both treatments, the number of buds with clearly differentiated leaf primordia increased in the course of 90 to 130 days, although it was only significantly greater in the treatments with NAA / BA (P <0.05), where the average of nine shoot buds observed at the beginning, nearly doubled at 130 days (Table 1); in contrast, in 2,4-D / K, these values remained practically unchanged (P <0.05).
Table 1 Adventitious buds formed from mature cygotic Pseudotsuga menziesii (Mirb.) Franco embryos after 90 and 130 days treated with 0.53/13.32 µM of NAA/BA or 2,4-D/K.
| Treatment | Embryos with buds (%) | Number of buds/embryo (± EE1) | Buds/hypocotyl (± EE) | Buds/cotyledons (± EE) | ||||
|---|---|---|---|---|---|---|---|---|
| 90 days | 130 days | 90 days | 130 days | 90 days | 130 days | |||
| NAA/BA | 75.8 | 9.1 ± 0.8 A | 16.2±1.4 A* | 4.1± 0.45 ab* | 7.8± 0.7 a* | 5 ± 0.45 a | 8.4± 0.8 a* | |
| 2,4-D/K | 73.7 | 5.9 ± 0.44 B | 7±0.36 B* | 3.12± 0.4 bc | 3.80± 0.25 b | 2.7 ± 0.2 c | 3.4 ± 0.22 b* | |
1 = Standard error. Capital letter indicates significant differences in the number of shoot buds between treatments at a given time (p <0.05). Lowercase letters indicate significant differences between embryo regions and treatment with growth regulators at a given time (P <0.05); * = Significant increase in the number of shoot buds over time in a treatment at a specific time (P <0.05).
Both the cotyledons and the hypocotyl contributed a similar number of shoot buds, although NAA / BA produced more (P <0.05); notwithstanding, the shoots formed from embryos treated with 2,4-D / K had appeared more vigorous, as they lengthened faster (Figure 1A and 1B).
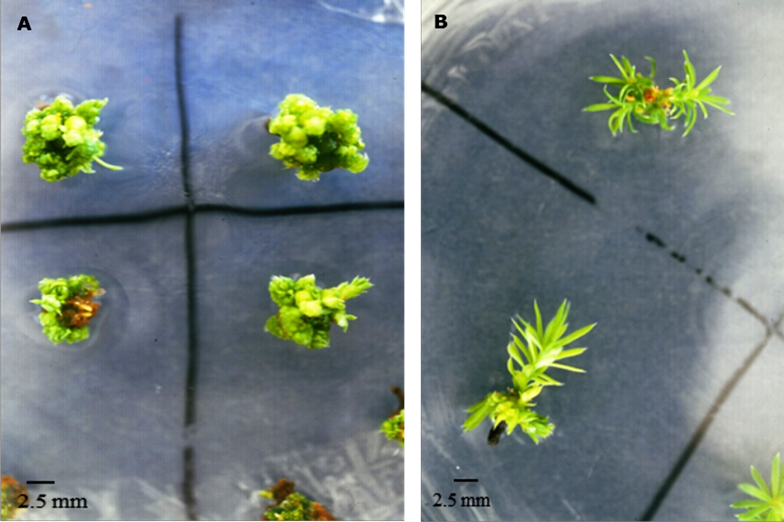
Figure 1 Adventitious buds formed after 130 days of cultivation treated with 0.53/13.32 µM A) NAA/BA or B) 2,4-D/K.
Additionally, the effect of the distance of the embryo tissues from the culture medium on its morphogenetic response was compared. The number of adventitious buds formed from cotyledons and hypocotyl of SCM was found to be similar for both treatments; around 37 % of the buds that grew on NAA/BA and 53 % in 2.4-D/K started from this surface. In NAA/BA a significant higher number of shoot buds from the SAM tissues were obtained (P< 0.05) (Table 2).
Table 2 Influence of the distance of the tissue from the culture medium over the average number of buds formed in cotyledons and hypocotyl of Pseudotsuga menziessi (Mirb.) Franco mature embryos after 90 days of cultivation.
| Induction treatment | Average number of buds/hypocotyl (± DE) | Average number of buds /cotyledons (± DE) |
|---|---|---|
| Surface in contact with medium (SCM) | ||
| NAA/BA | 1.63 ± 0.24 B | 1.65 ± 0.19 B |
| 2,4-D/K | 1.44 ± 0.20 B | 1.77 ± 0.18 B |
| Surface remote from the medium (SAM) | ||
| NAA/BA | 2.43 ± 0.29 A | 3.36 ± 0.34 A |
| 2,4-D/K | 1.67 ± 0.13 B | 1.00 ± 0.07 B |
Uppercase letters indicate significant differences between all the values (treatment and surface by treatment) (P <0.05).
Histological analysis
The histological study of the explants at different culture times showed the adventitious origin of the shoots by direct organogenesis. Although the developmental pattern was similar, the temporality of the appearance of the different structures varied between the treatments and tissue types (cotyledons vs. hypocotyl), which was delayed in 2,4-D / K and in the cotyledons. The reactivation of the embryo apical meristem was observed, from which an apical bud differentiated, while the root cap hypertrophied and degenerated over time.
The longitudinal sections of the embryos at the time of excision (Figure 2) showed that the epidermis has a single stratum of rectangular cells, longitudinally flattened, that surround the fundamental tissue and the central provascular strands. The cells of the fundamental tissue are parenchymatous, cubic, arranged neatly along the axis, with few intercellular spaces, have a conspicuous nucleus, and dense cytoplasm; while the provascular tissue region presented elongated nucleated cells. Both cell types contained abundant reserve material. The apical meristem of the shoot has the characteristic form of dome, formed by isodiametric cells, is densely cytoplasmic, with a prominent nucleus. At this stage, no mitotic activity was observed in any of the mentioned regions.
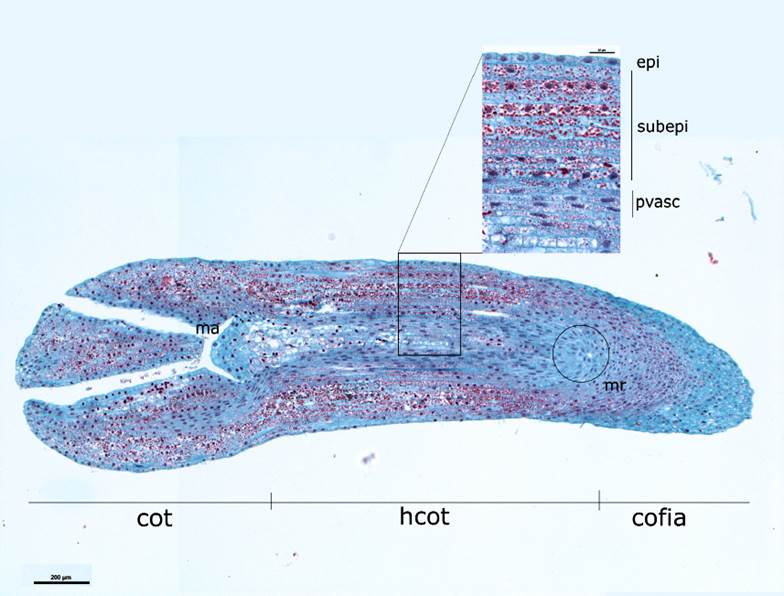
Cot = Cotyledons; hco = Hypocotyl; ma = Apical meristem; mr = Root meristem; epi = Epidermis; subepi = Subepidermis/ fundamental tissue; pvasc = Provascular beam.
Figure 2 Longitudinal cut of a Pseudotsuga menziesii (Mirb.) Franco mature embryo and close-up of the several cell layers that make it up.
The third and fourth days of culture were characterized by the reactivation of mitotic activity throughout the explant, with anticlinal cell divisions in the hypocotyl fundamental and provascular tissue, probably contributing to the lengthening of the embryo. Also, the appearance of vacuoles was noticed, as well as a decrease in the amount of reserve material in the fundamental tissue.
Between the fourth and the sixth day of culture, localized, periclinal and anticlinal cell divisions initiated in the subepidermal (Figure 3A) and epidermal (Figure 3B) layers of the embryonic axis, mainly in the intercotyledonary regions and in the mid-distal hypocotyl region. The response in the cotyledons was slower than in the hypocotyl and the cell divisions were scattered.
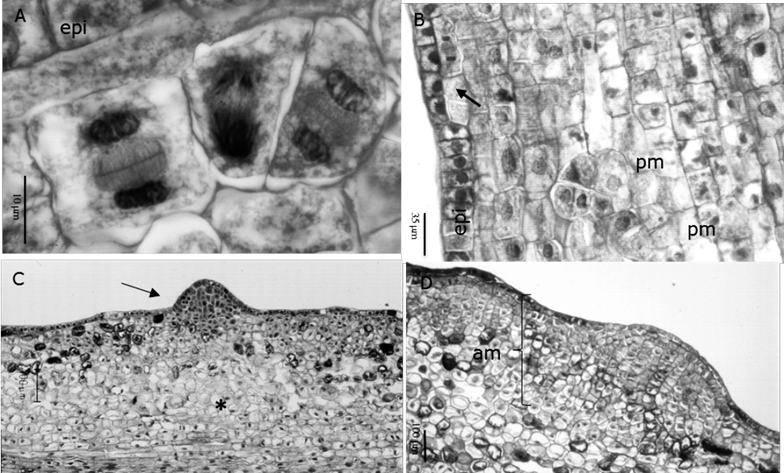
A.Formation of cell clusters in the subepidermal layer at six days (100X); B. Periclinal and anticlinal divisions of the epidermal layer (epi), as well as promeristemoids (pm) in the subepidermal layers (20X); C. Meristemoid in the hypocotyl of surface in contact with the medium after 14 days (10X); D. Meristematic region in the hypocotyl of the surface far from the medium after 16 days (10X).
Figure 3 Structural analysis of the early stages of adventitious shoot formation obtained from the in vitro culture of mature zygotic embryos of Pseudotsuga menziesii (Mirb.) Franco.
From the sixth day, both in NAA / BA and in 2,4-D / K, the morphogenetic pattern varied depending on the distance of the tissue from the culture medium. In the SAM, the first cell clusters and promeristemoids (structures of 4-6 cells) were observed (Figure 3B), which were initiated from the mitotic activation of one or very few cells and exhibited a scattered distribution throughout the fundamental tissue. These structures were first formed in the mid-distal hypocotyl region and subsequently extended to the cotyledons. After 12 days in NAA/BA and 14 days in 2.4-D / K, the first dome-shaped meristemoids were detected, which gave the surface of the embryo a nodular appearance (Figure 3C).
The epidermis formed part of these structures by donating cells through anticlinal divisions. The body of the meristemoids grew by means of periclinal and anticlinal divisions of cells ftom the fundamental tissue. In the cotyledons, the meristematic activity started after ten days of cultivation in NAA / BA, usually on the abaxial surface and at the tips; while in the 2,4-D / K treatments, it initiated after 16 to 22 days of culture and was limited to the tip.
On the other hand, extensive meristematic areas were formed in the SCM (Figure 3D), characterized by small cells of dense cytoplasm and prominent nucleus, that initiated from anticlinal and periclinal divisions of the epidermis, and anticlinal, periclinal and oblique divisions in the first two subepidermal layers. In NAA / BA and 2,4-D / K, this pattern began in the mid hypocotyl region after six days, and in the intercotyledonary regions after 10 to 12 days; wheras the cotyledons responded belatedly at 14 and 16 days in NAA / BA and 2,4-D / K, respectively. After 16 days meristematic areas reached a width of eight to ten cells and covered the total length of the embryo (except the root cap), giving the embryo surface an irregular appearance, even if the cells remained relatively organized and contained by the epidermis (Figure 4A). From 22 to 45 days of culture, these areas originated nodular structures of meristematic appearance, from which apical buds were differentiated. Although in most cases the epidermis of the embryo became part of the nodule, other structures were observed, possibly proembryos, that emerged to the surface after breaking the epidermis (Figure 4B).
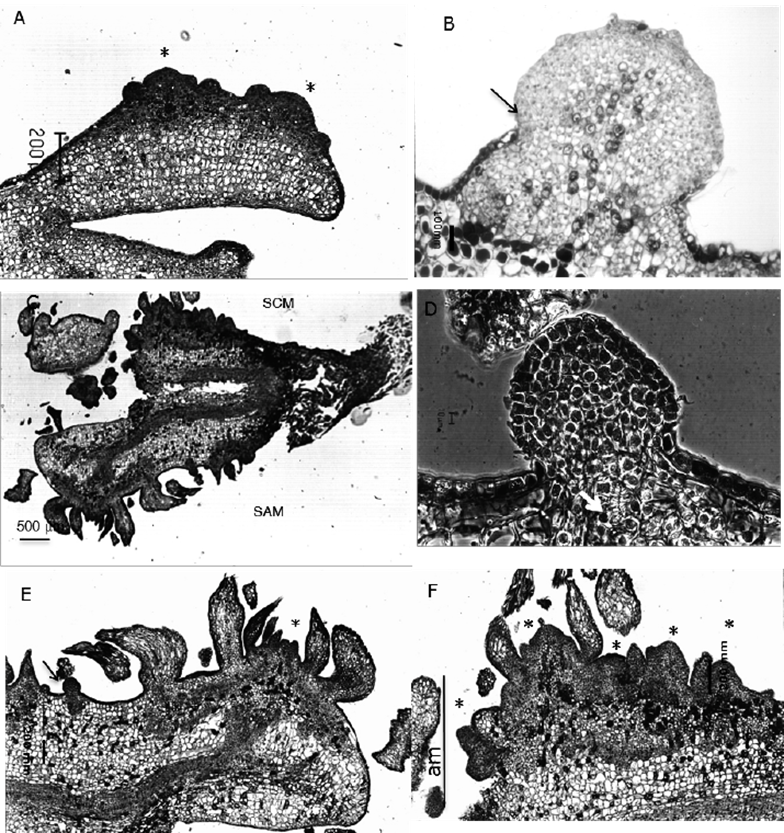
A. Bud primordia formed on cotyledons after 30 days (4X); B. Meristematic nodular structure formed on the hypocotyl surface in contact with the media (SCM) after 30 days; the arrow indicates the rupture of the epidermis (10X); C. Bud formation along the hypocotyl and cotyledons after 40 days (5X); D. Meristematic nodule showing tracheid differentiation (arrow), Normanski 50X; E. Close up of a shoot buds forming on a cotyledon on the surface away from the medium (SAM), as well as meristematic nodule (arrow) (10X); F. Close up of buds forming from a meristematic area (am) of the surface in contact with the medium (SCM) (20X).
Figure 4 Late stages of adventitious shoot formation in cultured Pseudotsuga menziesii (Mirb.) Franco embryos.
The first shoots (formed by an apical dome with leaf primordia) were distinguished in the hypocotyl after 14 days, although the process became generalized between a 20 and 30 day period in 2,4-D / K and NAA / BA, respectively; whereas in the cotyledons, these lagged behind and were apparent only after 35 to 40 days. Longitudinal sections at 45 days, showed that even though many shoots were clearly formed, differentiation was asynchronous, particularly in NAA / BA, where it was still possible to identify meristemoids or primordia at early developmental stages, which prolonged the initiation and bud growth period, explaining the notorious increase in shoot bud number between 90 and 130 days (Table 2).In contrast, in explants subjected to 2,4-D / K, meristemoids were rarely seen in later stages of culture.
The initiation of shoot buds from the meristematic nodules was documented after 60 days in culture (Figure 4C -F), indicating their morphogenetic capacity. The accumulation of condensed tannins around the meristemoids and buds was identified, as well as the de novo formation of associated tracheids, even before the leaf primordia were evident; however, they rarely connected to the embryonic vascular system.
In the present work, cellular changes indicative of the loss of competence, and therefore, the beginning of recalcitrance, were also noticed. From day six of cultivation, cells that did not respond meristematically, formed large vacuoles inside, and displaced the pressed nucleus against the cell wall. In late stages of cultivation, they presented a loose and irregular arrangement and the accumulation of polysaccharides in the surrounding extracellular matrix, likewise, many of them, especially in the epidermis and near the meristemoids and meristematic zones, stored tannins inside.
Discussion
In vitro culture of complete zygotic embryos represents a viable alternative to propagation of Mexican genotypes of P. menziesii as it increases the number of shoot buds, and potentially the number of plants, that can be obtained from a single embryo. Galindo et al. (2000) indicate that organogenesis from the embryos of this species occurs within a relatively broad range of auxin and cytokinin concentrations, both in NAA / BA and 2,4-D / K, although the number of buds, survival and their growth speed differs.
The ability to respond morphogenetically was similar for both treatments, and started in epidermal and subepidermal tissues, both hypocotyl and cotyledons. In general, this contrasts with other studies with Pseudotsuga, in which only cotyledons of in vitro germinated embryos are used as the main explant (Cheng and Voqui, 1977). The results of the present work show that the management of the complete zygotic embryo allows the use of different ontogenetically predetermined regions for growth, which keeps the cells in a competent state and may be a useful option for in vitro propagation in recalcitrant genotypes or species.
It was confirmed that the use of BA promoted the formation of a greater number of shoots, although in the treatment with K, they acquired a more vigorous appearance. Similar data were verified for other species with which the effect of different types of cytokinins on the growth of adventitious shoots was compared, including genotypes of P. menziesii from the United States of America (Cheng and Voqui, 1977), as well as several species of Pinus (Pelletier and Laliberté, 2000; Montalbán et al., 2011) and Picea (López et al., 2000).
The lower number of shoots observed at 2,4-D / K after 130 days of culture means that the period during which the cells of the explant remain competent is lower than in the cultures of NAA / BA. This was corroborated with the histological analysis, through which it was possible to demonstrate that in NAA / BA the explants produce meristemoids even in later stages of culture. Although the causes of this response are not known, in other systems it has been reported that the ability of the explants to absorb and metabolize cytokinins varies according to the type of regulator, mainly citokinines, the culture time and the development of the explant (Cortizo et al., 2009). It can not be ruled out that the use of 2,4-D, considered an inducer of callus and embryogenesis, could influence a more rapid loss of competence, particularly in cotyledons.
The formation of adventitious shoots followed the described pattern (Cheah and Cheng 1978; Yeung et al., 1981; Villalobos et al., 1985; von Arnold and Grönroos, 1986; López et al., 2000) that began with the mitotic reactivation of one or two cells that promoted, in sequential order, promeristemoides, meristemoides and then the apical dome integrated by the meristem and the leaf primordia.
However, the spatial distribution and temporal asynchrony of the morphogenetic process evidenced the importance of the position of the cells with respect to the culture medium for their competence. Although buds developed both on the surface away from the medium, and in the that in direct contact with it, in the former, they started from individual meristemoids, while in the latter, in NAA / BA in particular, large meristematic zones developed that differentiated buds in later stages of cultivation. This is consistent with the hypothesis that the establishment of hormonal and nutrient gradients in the explants is an important factor in the induction of cellular competence and subsequent organogenic determination (Yeung et al., 1981; Joy and Thorpe, 1999).
According to von Arnold and Grönroos (1986) meristematic areas are the result of supra-optimal and prolonged exposure to cytokinins, so the formation of distinctive meristemoids is obtained by reducing the exposure time to these growth regulators. In Pinus banksiana Lamb., Pelletier and Laliberté (2000) described that cells in contact with the medium proliferate but never give rise to buds, which contrasts with the findings of the present work.
Finally, the majority of the buds formed had a multicellular origin, since meristemoids were constituted by cells donated by the epidermis as well as by the subepidermis. This contrasts with previous reports of the development of buds from cotyledon explants of P. menziesii, which displayed a subepidermal origin and emerged by rupturing the epidermis (Cheah and Cheng, 1978), suggesting a loss of epidermal competence in differentiated tissue or with a higher degree of maturation.
In the current study, the epidermis experienced significant mitotic activity, with anticlinal and periclinal divisions, which donated cells to both the embryo surface and the underlying tissue and the meristematic regions under development. Such behavior has been observed in micropropagation systems proposed for Pinus radiata D. Don (Villalobos et al, 1985), Pinus strobus L. (Flinn et al., 1988) and Abies amabilis Douglas ex Forbes (Kulchetscky et al., 1995). Although this type of reaction was stimulated with the induction treatments, it is possible that it was also facilitated by a predisposition of the epidermis to respond meristematically, a process that Allen (1947) described in one of the few published works on zygotic embryogenesis for the gender. The multicellular origin of the structures can have a strong impact on future breeding strategies, particularly considering ways such as genetic transformation, since the obtaining of chimeras is highly probable.
Conclusions
This work follows the initiation and early stages of adventitious bud formation from the culture of complete mature zygotic embryos of P. menziesii, in response to the treatments with NAA / BA and 2,4-D / K. Histological analysis contributes to the understanding of the cellular events that precede the generation of new organs, as well as the loss of morphogenetic competence and the beginning of recalcitrance.
The number of buds formed in NAA / BA is higher than in 2,4-D / K, although the process was more asynchronous. The histological analysis shows that the caulogénesis process occurs via direct organogenesis and the shoots have a multicellular origin, since they are formed by cells of both the epidermis and the subepidermal cell layers, which gives rise to meristematic or meristemoid zones, depending on their distance of the culture medium.
The loss of cellular competition occurs first in 2,4-D / K, and the greater number of buds formed in NAA / BA results from a prolonged meristematic activity.
These kind of studies constitute the basis of future research for the optimization of culture techniques with important implications by providing a structural context to address the physiological and molecular events that regulate morphogenesis both in vitro and ex vitro.
Acknowledgements
Florencia García had a grant from the Dirección General de Estudios de Posgrado (DGEP) (General Direction of Graduate Studies) of UNAM (National University of Mexico) and Guadalupe Monjarás from the Consejo Nacional de Ciencia y Tecnología (National Science and Technoloy Council). This work was financed by the Sistema de Investigación Ignacio Zaragoza (SIZA)-Conacyt (970603003) and by the Fundación Produce-Tlaxcala (UAT-06). The authors also thank the anonymous reviewers for their observations which helped to improve the manuscript.
REFERENCES
Allen, G. S. 1947. Embryogeny and the development of the apical meristems of Pseudotsuga. II. Late embryogeny. American Journal of Botany 34(2): 73-80. Doi: 10.1002/j.1537-2197.1947.tb12960.x. [ Links ]
Bonga, J. M. 2015. A comparative evaluation of the application of somatic embryogenesis, rooting of cuttings, and organogenesis of conifers. Canadian Journal of Forest Research 45(4): 379-383. Doi: 10.1139/cjfr-2014-0360. [ Links ]
Carrillo B., M. G., J. L. Rodríguez De la O y J. G. Álvarez M. 2011. Morfogénesis in vitro de Pseudotsuga menziesii var. glauca. Revista Chapingo, Serie Ciencias Forestales y del Ambiente 17(2): 273-282. Doi: 10.5154/r.rchscfa.2010.04.024. [ Links ]
Cheah, K. T. and T. Y. Cheng. 1978. Histological analysis of adventitious bud formation in cultured Douglas-fir cotyledon. American Journal of Botany 65: 845-849. Doi: 10.2307/2442179. [ Links ]
Cheng, T. Y. and T. H. Voqui. 1977. Regeneration of Douglas-fir plantlets through tissue culture. Science 198(4314): 306-307. Doi: 10.1126/science.198.4314.306. [ Links ]
Cortizo, M., C. Cuesta, M. L. Centeno, A. Rodríguez, B. Fernández and R. Ordás. 2009. Benzyladenine metabolism and temporal competence of Pinus pinea cotyledons to form buds in vitro. Journal of Plant Physiology 166(10): 1069-1076. Doi: 10.1016/j.jplph.2008.12.013. [ Links ]
Durzan, D. J. and P. K. Gupta. 1987. Somatic embryogenesis and polyembriogenesis in Douglas-fir cell suspension cultures. Plant Science 52(3): 229-235. Doi: 10.1016/0168-9452(87)90056-2. [ Links ]
Flinn, B. S., D. T. Webb and W. Newcomb. 1988. The role of cell clusters and promeristemoids in determination and competence for caulogenesis by Pinus strobus cotyledons in vitro. Canadian Journal of Botany 66(8): 1556-1565. Doi: 10.1139/b88-214. [ Links ]
Galindo F., G., F. García C., G. Monjarás G. y V. M. Chávez Á. 2000. Regeneración in vitro de Pseudotsuga macrolepis Flous a partir de embriones maduros. Investigación para el Desarrollo Regional. SEP-CONACYT. México, D. F., México. pp. 165-169. [ Links ]
Gugger, P. F., A. González, H. Rodríguez, S. Sugita and J. Cavender B. 2011. Southward Pleistocene migration of Douglas-fir into Mexico: phylogeography, ecological niche modeling and conservation of ‘rear edge’ populations. New Phytologist 189:1185-1199. Doi: 10.1111/j.1469-8137.2010.03559. [ Links ]
Gupta, P. K. and D. J. Durzan. 1985. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Reports 4(4): 177-179. Doi: 10.1007/BF00269282. [ Links ]
Hermann, R. K. and D. P. Lavender. 1990. Pseudotsuga menziesii (Mirb.) Franco: Pinaceae. In: Russel, M. B. and B. H. Honkala (eds.) Silvics of North America. Vol 1: Conifers. Agriculture Handbook 654. Vol.1. USDA Forest Services. Washington D.C., USA. pp. 527-540. [ Links ]
Joy, R. W. and T. A. Thorpe. 1999. Shoot morphogenesis: structure, physiology, biochemistry and molecular biology. In: Soh, W. and S. S. Bhojwani (eds.). Morphogenesis in plant tissue cultures. Springer. The Netherlands. pp. 171-214. [ Links ]
Juárez A., A., J. López U., J. J. Vargas H. and C. Sáenz R. 2006. Geographic variation in germination and initial seedling growth of Pseudotsuga menziesii of México. Agrociencia 40: 783-792. [ Links ]
Kulchetscky, L., I. S. Harry, E. C. Yeung and T. A. Thorpe. 1995. In vitro regeneration of Pacific silver fir (Abies amabilis) plantlets and histological analysis of shoot formation. Tree Physiology 15(11): 727-738. Doi: 10.1093/treephys/15.11.727. [ Links ]
López, A. L., L. P. Olguín, J. Márquez, V. M. Chávez and R. Bye. 2000. Adventitious bud formation from mature embryos of Picea chihuahuana Martínez, an endangered Mexican spruce tree. Annals of Botany 86(2): 921-927. Doi: 10.1006/anbo.2000.1257 [ Links ]
Mápula, L., M., R. Bonilla y D. A. Rodríguez. 1996. Germinación y crecimiento inicial de Pseudotsuga macrolepis Flous en Chapingo, México. Revista Chapingo: Ciencias Forestales 5: 111-117. [ Links ]
Montalbán, I. A., N. De Diego and P. Moncaleán. 2011. Testing novel cytokinins for improved in vitro adventitious shoots formation and subsequent ex vitro performance in Pinus radiata. Forestry 84(4): 363-373. Doi: 10.1093/forestry/cpr022. [ Links ]
Pelletier, G. and S. Laliberté. 2000. Effect of embryo orientation on the developmental sequence of adventitious organogenesis in jack pine (Pinus banksiana Lamb). Canadian Journal of Botany 78(10): 1348-1360. Doi: 10.1139/b00-105 [ Links ]
R Development Core Team. 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org. [ Links ]
Schenk, R. U. and A. C. Hildebrandt. 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian Journal of Botany 50(1): 199-204. Doi: 10.1139/b72-026. [ Links ]
Secretaría de Medio Ambiente y Recursos Naturales (Semarnat). 2010. Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. 30 de diciembre de 2010. Diario Oficial de la Federación. México, D.F., México. 40 p. [ Links ]
Vargas, J. J., J. López U., V. J. Reyes, A. Domínguez and M. Mápula. 2004. Natural populations of Douglas- fir in Mexico: current status and needs for conservation. In: Beaulieu J (ed.). Silviculture and the conservation of genetic resources for sustainable forest management. In: Proceedings of the symposium of the North American Forest Commission: forest genetic resources and silviculture working groups, and the International Union of Forest Research Organizations (IUFRO). Quebec City, QC, Canada. pp. 26-36. [ Links ]
Velasco G., M. V., J. López U., G. Ángeles P., J. J. Vargas H. and V. Guerra-de la C. 2007. Pseudotsuga menziesii seed dispersion in populations of central Mexico. Agrociencia 41:121-131. [ Links ]
Villalobos, V. M., E. C. Yeung and T. A. Thorpe. 1985. Origin of adventitious shoots in excised radiata pine cotyledons cultured in vitro. Canadian Journal of Botany 63(12): 2172-2176. Doi: 10.1139/b85-307 [ Links ]
von Arnold, S. and R. Grönroos. 1986. Anatomical changes and peroxidase activity after cytokinin treatments inducing adventitious bud formation on embryos of Picea abies. Botanical Gazette 147(4): 425-431. Doi: 10.1086/337610 [ Links ]
Winton, L. L. and S. A. Verhagen. 1977. Shoots from Douglas-fir cultures. Canadian Journal of Botany 55 (9): 1246-1250. Doi: 10.1139/b77-144 [ Links ]
Yeung, E. C., J. Aitken, S. Biondi and T. A. Thorpe. 1981. Shoot histogenesis in cotyledon explants of radiate pine. Botanical Gazette 142:494-501. Doi: 10.1086/337251. [ Links ]
Zavala C., F. y J. T. Méndez M. 1996. Factores que afectan la producción de semillas en Pseudotsuga macrolepis Flous. en el Estado de Hidalgo, México. Acta Botánica Mexicana 36: 1-13. Doi: 10.21829/abm36.1996.756. [ Links ]
Received: March 14, 2019; Accepted: June 17, 2019





![Identificación del agente causal de la antracnosis en el cultivo de hule [Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg.]](/img/es/next.gif)





 texto en
texto en 



